
- May. 15, 2025
- Home
- About Us
- Editorial Board
- Instruction
- Subscription
- Advertisement
- Contact Us
- Chinese
- RSS

Chinese Journal of Magnetic Resonance ›› 2021, Vol. 38 ›› Issue (2): 239-248.doi: 10.11938/cjmr20202849
• Articles • Previous Articles Next Articles
Meng-yu DOU1,2,Qi ZHAO1,2,Xiang-lin HOU1,2,Lei LIU3,Ming-xing TANG1,*( ),Ying-xiong WANG1,2,*(
),Ying-xiong WANG1,2,*( )
)
Received:2020-08-24
Online:2021-06-05
Published:2020-09-24
Contact:
Ming-xing TANG,Ying-xiong WANG
E-mail:mxtang@sxicc.ac.cn;wangyx@sxicc.ac.cn
CLC Number:
Meng-yu DOU,Qi ZHAO,Xiang-lin HOU,Lei LIU,Ming-xing TANG,Ying-xiong WANG. Structural Elucidation and Quantitative Analysis of Hydrogenation Products of Anthracene by NMR Spectroscopy[J]. Chinese Journal of Magnetic Resonance, 2021, 38(2): 239-248.
Table 1
1H NMR and 13C NMR data assignments of anthracene hydrogenation products
| 化合物 | 分子结构 | 原子编号 | δC | δH (J/Hz) | HSQC | 1H-1H COSY |
| 二氢蒽 | 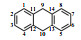 | 1/4/5/8 | 127.4 | 7.29 (4H, dd, J=5.5/3.4) | + | H-2/3/6/7 |
| 2/3/6/7 | 126.1 | 7.19 (4H, dd, J=5.6/3.3) | + | H-1/4/5/8 | ||
| 9/10 | 36.1 | 3.93 (4H, s) | + | / | ||
| 11/12/13/14 | 136.8 | / | / | / | ||
| 四氢蒽 | 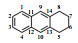 | 1/4 | 124.9 | 7.34 (2H, dd, J=6.3/3.2) | + | H-2/3 |
| 2/3 | 127.0 | 7.70 (2H, dd, J=6.2/3.3) | + | H-1/4 | ||
| 6/7 | 23.4 | 1.86 (4H, m) | + | H-5/8 | ||
| 5/8 | 29.9 | 2.97 (4H, m) | + | H-6/7 | ||
| 9/10 | 126.6 | 7.53 (2H, s) | + | / | ||
| 11/12 | 136.2 | / | / | / | ||
| 13/14 | 136.1 | / | / | / | ||
| 对称八氢蒽 | 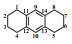 | 1/4/5/8 | 29.0 | 2.70 (8H, m) | + | H-2/3/6/7 |
| 2/3/6/7 | 23.4 | 1.77 (8H, m) | + | H-1/4/5/8 | ||
| 9/10 | 129.7 | 6.78 (2H, s) | / | / | ||
| 11/12/13/14 | 129.5 | / | / | / | ||
| 蒽* | 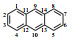 | 1/4/5/8 | 128.2 | 8.00 (4H, dd, J=6.4/3.3) | + | H-2/3/6/7 |
| 2/3/6/7 | 125.4 | 7.45 (4H, dd, J=6.6/3.3) | + | H-1/4/5/8 | ||
| 9/10 | 126.2 | 8.42 (2H, s) | + | / | ||
| 11/12/13/14 | 131.7 | / | / | / |
Table 2
Quantitative analysis for QNMR tests of the 3 samples
| 化合物 | DHA | THA | sym-OHA | (cis+trans) OHA | AN | 吡嗪 | |
| 分子量 | 180.09 | 182.11 | 186.14 | 186.14 | 178.08 | 80.04 | |
| 积分范围 | 7.27~7.32 | 7.68~7.73 | 6.78~6.80 | 7.02~7.10 | 8.41~8.44 | 8.58~8.61 | |
| 质子数 | 4 | 2 | 2 | 4 | 2 | 4 | |
| 样品1 | 含量/mg | 1.59 | 1.34 | 0.20 | 0 | 0 | 0.18 |
| 选择性/% | 50.73 | 42.80 | 6.47 | / | / | / | |
| 转化率/% | / | / | / | / | 100 | / | |
| 相对偏差/% | 0.82 | 2.18 | 1.93 | / | / | / | |
| 样品2 | 含量/mg | 0.62 | 1.33 | 0.08 | 0.07 | 0.06 | 0.18 |
| 选择性/% | 29.39 | 63.28 | 3.74 | 3.59 | / | / | |
| 转化率/% | / | / | / | / | 92.27 | / | |
| 相对偏差/% | 1.37 | 0.20 | 1.69 | 7.41 | 2.62 | / | |
| 样品3 | 含量/mg | 2.98 | 0.22 | 2.99 | 1.84 | 0 | 0.18 |
| 选择性/% | 37.29 | 2.70 | 36.93 | 23.08 | / | / | |
| 转化率/% | / | / | / | / | 100 | / | |
| 相对偏差/% | 2.11 | 4.28 | 1.78 | 2.37 | / | / |
| 1 |
YUAN T , MARSHALL W D . Catalytic hydrogenation of polycyclic aromatic hydrocarbons over palladium/gamma-Al2O3 under mild conditions[J]. J Hazard Mater, 2005, 126 (1-3): 149- 157.
doi: 10.1016/j.jhazmat.2005.06.022 |
| 2 |
FANG D , WANG G , LIU M J , et al. Combined selective hydrogenation and catalytic cracking process for efficient conversion of heavy cycle oil to high octane number gasoline[J]. Ind Eng Chem Res, 2019, 58 (43): 19752- 19759.
doi: 10.1021/acs.iecr.9b03896 |
| 3 | JACINTO M J , SANTOS O H C F , LANDERS R , et al. On the catalytic hydrogenation of polycyclic aromatic hydrocarbons into less toxic compounds by a facile recoverable catalyst[J]. Appl Catal B-Environ, 2009, 90 (3, 4): 688- 692. |
| 4 |
PETRUKHINA N N , VINNIKOVA M A , MAKSIMOV A L . Production of high-density jet and diesel fuels by hydrogenation of highly aromatic fractions[J]. Russ J Appl Chem, 2018, 91, 1223- 1254.
doi: 10.1134/S1070427218080013 |
| 5 | KOTANIGAWA T , YAMAMOTO M , YOSHIDA T . Selective nuclear hydrogenation of naphthalene, anthracene and coal-derived oil over Ru supported on mixed oxide[J]. Appl Catal A-Gen, 1997, 164 (1, 2): 323- 332. |
| 6 |
PINILLA J L , GARCíA A B , PHILIPPOT K , et al. Carbon-supported Pd nanoparticles as catalysts for anthracene hydrogenation[J]. Fuel, 2014, 116, 729- 735.
doi: 10.1016/j.fuel.2013.08.067 |
| 7 | PAL S , MUKHERJEE M , PODDER D , et al. A highly regioselective 6-endo-aryl radical cyclisation: stereocontrolled synthesis of trans-octa hydroanthracenes[J]. J Chem Soc Chem Commun, 1991, 22, 1591- 1593. |
| 8 |
JACINTO M J , WIZBIKI M , JUSTINO L C , et al. Platinum-supported mesoporous silica of facile recovery as a catalyst for hydrogenation of polyaromatic hydrocarbons under ultra-mild conditions[J]. J Sol-Gel Sci Techn, 2015, 77 (2): 298- 305.
doi: 10.1007/s10971-015-3854-6 |
| 9 |
BAIKENOV M I , MEIRAMOV M G , KHALIKOVA Z S , et al. Catalytic hydrogenation of anthracene in ethanol[J]. Solid Fuel Chem, 2016, 50 (4): 256- 259.
doi: 10.3103/S0361521916040029 |
| 10 |
JACINTO M J , GONZALES M G , ZANATO A F S , et al. Rh nanoparticles grafted on mesoporous silica support as a high-efficiency catalyst for Anthracene hydrogenation[J]. Sustain Chem Pharm, 2017, 6, 90- 95.
doi: 10.1016/j.scp.2017.10.002 |
| 11 |
KALENCHUK A N , KOKLIN A E , BOGDAN V I , et al. Hydrogenation of anthracene and dehydrogenation of perhydroanthracene on Pt/C catalysts[J]. Russ J Phys Chem A, 2018, 92 (4): 663- 668.
doi: 10.1134/S0036024418040106 |
| 12 | 大连理工大学. 一种工业蒽选择加氢-氧化耦合制均苯四甲酸二酐的方法与流程: 中国, 10375903[P], 2018-10-12 |
| 13 | 大连理工大学. 一种蒽选择加氢制对称八氢蒽的方法与流程: 中国, 10316649[P], 2018-09-28 |
| 14 |
BOTTOMLEY P A . NMR imaging techniques and applications: a review[J]. Rev Sci Instrum, 1982, 53 (9): 1319- 1337.
doi: 10.1063/1.1137180 |
| 15 |
MCKENZIE J S , DONARSKI J A , WILSON J C , et al. Analysis of complex mixtures using high-resolution nuclear magnetic resonance spectroscopy and chemometrics[J]. Prog Nucl Mag Res Sp, 2011, 59 (4): 336- 359.
doi: 10.1016/j.pnmrs.2011.04.003 |
| 16 | WANG L M , QIU R C , HUANG S H . Quantitative analysis of active ingredients in compound acetylsalicylic acid tablets by DOSY[J]. Chinese J Magn Reson, 2016, 33 (3): 415- 421. |
| 王丽敏, 仇汝臣, 黄少华. 复方乙酰水杨酸片中有效成分的DOSY技术分析[J]. 波谱学杂志, 2016, 33 (3): 415- 421. | |
| 17 |
DAL POGGETTO G , CASTAñAR L , MORRIS G A , et al. A new tool for NMR analysis of complex systems: selective pure shift TOCSY[J]. RSC Adv, 2016, 6 (102): 100063- 100066.
doi: 10.1039/C6RA22807K |
| 18 |
MACKINNON N , WHILE P T , KORVINK J G . Novel selective TOCSY method enables NMR spectral elucidation of metabolomic mixtures[J]. J Magn Reson, 2016, 272, 147- 157.
doi: 10.1016/j.jmr.2016.09.011 |
| 19 | YAN K , BAI Z W , HUANG S H . NMR signal separation of ionic liquids by poly(sodium-p-styrenesulfonate)-assisted chromatographic NMR spectroscopy[J]. Chinese J Magn Reson, 2019, |
| 严葵, 柏正武, 黄少华. 利用聚(苯乙烯磺酸钠)辅助的DOSY技术分析离子液体[J]. 波谱学杂志, 2019, | |
| 20 |
LYU Z X , YUE F , YAN X Y , et al. Combination of DOSY and 1D selective gradient TOCSY: Versatile NMR tools for identify the mixtures from glycerol hydrogenolysis reaction[J]. Fuel Process Technol, 2018, 171, 117- 123.
doi: 10.1016/j.fuproc.2017.11.011 |
| 21 |
MA H , PEDERSEN C M , ZHAO Q , et al. NMR analysis of the Fischer-Tropsch wastewater: Combination of 1D selective gradient TOCSY, 2D DOSY and qNMR[J]. Anal Chim Acta, 2019, 1066, 21- 27.
doi: 10.1016/j.aca.2019.04.007 |
| 22 |
ZHAO Q , LIU Y , MA H , et al. Combination of pure shift NMR and chemical shift selective filters for analysis of Fischer-Tropsch waste-water[J]. Anal Chim Acta, 2020, 1110, 131- 140.
doi: 10.1016/j.aca.2020.03.014 |
| 23 |
LYU Z X , GAO F E , WEN L Z , et al. DOSY Plus Selective TOCSY: An efficient NMR combination for analyzing hydrogenation/hydrogenolysis mixtures of biomass-derived platform compounds[J]. Energ Fuels, 2018, 32 (3): 3551- 3558.
doi: 10.1021/acs.energyfuels.7b03992 |
| 24 |
RUNDLOF T , MATHIASSON M , BEKIROGLU S , et al. Survey and qualification of internal standards for quantification by 1H NMR spectroscopy[J]. J Pharmaceut Biomed, 2010, 52 (5): 645- 651.
doi: 10.1016/j.jpba.2010.02.007 |
| 25 | ZHANG F F , SHEN W B , XU K B , et al. A proton nuclear magnetic resonance method for quantitative analysis of ticagrelor[J]. Chinese J Magn Reson, 2020, 37 (2): 216- 223. |
| 张芬芬, 沈文斌, 徐开兵, 等. 定量核磁共振氢谱测定新药替格瑞洛[J]. 波谱学杂志, 2020, 37 (2): 216- 223. | |
| 26 |
TAVERNIER D , ANTEUNIS M J O . Stereochemical aspects of proton chemical shifts. X-the slow exchange 1H NMR spectrum of cis-transoid-cis-perhydroanthracene and of cis-decalin[J]. Organ Magn Reson, 1982, 18 (2): 109- 111.
doi: 10.1002/mrc.1270180213 |
| 27 |
BARJAT H , MORRIS G , SMART S , et al. High-resolution diffusion-ordered 2D spectroscopy (HR-DOSY)-A new tool for the analysis of complex mixtures[J]. J Magn Reson B, 1995, 108 (2): 170- 172.
doi: 10.1006/jmrb.1995.1118 |
| 28 | JR C S J . Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications[J]. Prog Nucl Mag Res Sp, 1999, 34 (3, 4): 203- 256. |
| 29 |
FACKE T , BERGER S . Application of pulsed field gradients in an improved selective TOCSY experiment[J]. J Magn Reson A, 1995, 113 (2): 257- 259.
doi: 10.1006/jmra.1995.1090 |
| 30 |
LI W Y , ZHENG H , YE C P , et al. Effect of the intermolecular hydrogen bond between carbazole and N, N-dimethylformamide/isopropanolamine on the solubility of carbazole[J]. Energ Fuels, 2012, 26 (10): 6316- 6322.
doi: 10.1021/ef301240t |
| 31 | WANG M , HUI Y H , ZHANG X H , et al. Oxidation of tetrahydrofuran[J]. Progress in Chemistry, 2013, 25 (7): 1158- 1165. |
| 王猛, 惠永海, 张雪华, 等. 四氢呋喃的氧化[J]. 化学进展, 2013, 25 (7): 1158- 1165. |
| [1] | Zi-hao WANG,He XU,Tao WANG,Shan-zhong YANG,Yun-sheng DING,Hai-bing WEI. NMR Spectroscopic Studies on (exo, endo) C-2 Monosubstituted Norbornene Derivatives [J]. Chinese Journal of Magnetic Resonance, 2021, 38(3): 323-335. |
| [2] | Xiao-li CHEN,Tian-qiao YONG,Cheng CHEN,Juan FU,Jia-mei MO,Qiu-cheng SU. Physical and Chemical Properties of Silicone Electrolyte Materials Evaluated by Nuclear Magnetic Resonance Technology [J]. Chinese Journal of Magnetic Resonance, 2021, 38(3): 291-300. |
| [3] | HUANG Shan-shan, YAO Ye-feng, LI Peng, HE Pei-zhong. Quantum Chemical Calculation and Simulation of HSQC Experiments in Liquid-State NMR [J]. Chinese Journal of Magnetic Resonance, 2021, 38(1): 32-42. |
| [4] | WANG Rui-di, XU Bei-bei, SONG Yan-hong, WANG Xue-lu, YAO Ye-feng. Methanol-Water Interaction in Photocatalytic Methanol Reforming ─ An Operando NMR Study [J]. Chinese Journal of Magnetic Resonance, 2021, 38(1): 43-57. |
| [5] | ZHOU Zhong-gao, XIE Qian, YUAN Yang-yang, LI Jing, LU Dong-liang, CHEN Zheng-wang. An NMR Study of Chiral Glucopyranosyl-Based N-Heterocyclic Carbene-Palladium(II)-Pyridine Complex [J]. Chinese Journal of Magnetic Resonance, 2020, 37(4): 505-514. |
| [6] | WANG Si-hong, ZHANG Jing-dong, YIN Xiu-mei, LI Dong-hao, KAN Yu-he, HU Wei. NMR Assignments of 6-(4-chlorophenoxy)-tetrazolo[5,1-a]phthalazine [J]. Chinese Journal of Magnetic Resonance, 2020, 37(3): 390-398. |
| [7] | YANG Yi-ning, WANG Xue-lu, YAO Ye-feng. The Effects of Reaction Environment on Photocatalytic Methanol Reforming Studied by Operando Nuclear Magnetic Resonance Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2020, 37(1): 104-113. |
| [8] | LIN Xiao-qing, LI Hong, ZHAN Hao-lin, DU Shi-jia, HUANG Yu-qing, CHEN Zhong. High-Resolution Pure Shift NMR Spectroscopy and Its Applications [J]. Chinese Journal of Magnetic Resonance, 2019, 36(4): 425-436. |
| [9] | LIU Ji-hong, JIN Kun, WANG Ping, LUO Gen. An NMR Study on Esculetin and It's Derivatives [J]. Chinese Journal of Magnetic Resonance, 2019, 36(3): 341-349. |
| [10] | LI Hong-wei, YUAN Zhi-liang, XIA Bin. Determination of Apparent Protein Molecular Weight in Solution by Diffusion Ordered NMR Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2018, 35(3): 280-286. |
| [11] | HUANG Jun-lin, YU Yi-hua. Effects of Digital Resolution on Diffusional Dimension in DOSY Experiments [J]. Chinese Journal of Magnetic Resonance, 2018, 35(3): 287-293. |
| [12] | MA Lin-ge, LONG Yin-hua. Determination of Aromatic Carbon Mole Ratio in Oil Samples by Quantitative 13C NMR Spectroscopy with DFSS Methodology [J]. Chinese Journal of Magnetic Resonance, 2017, 34(1): 8-15. |
| [13] | WANG Li-min, QIU Ru-chen, HUANG Shao-hua. Quantitative Analysis of Active Ingredients in Compound Acetylsalicylic Acid Tablets by DOSY [J]. Chinese Journal of Magnetic Resonance, 2016, 33(3): 415-421. |
| [14] | HE Bao-jiang1, XU Xiu-juan1, YANG Wei-ping1, ZHANG Wen-juan1, WU Rui3, HUANG Shao-hua2*, YANG Ying2, BAI Zheng-wu3*. Progresses in Matrixed Chromatographic NMR [J]. Chinese Journal of Magnetic Resonance, 2015, 32(4): 699-706. |
| [15] | WU Rui1,BAI Zheng-wu1*,YANG Ying2,HUANG Shao-hua2*. Evaluation of Separation Performance of Polydimethylsiloxane-Matrixed Chromatography by NMR [J]. Chinese Journal of Magnetic Resonance, 2015, 32(3): 511-517. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 368
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 354
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||