
波谱学杂志 ›› 2021, Vol. 38 ›› Issue (4): 460-473.doi: 10.11938/cjmr20212913
收稿日期:2021-04-28
出版日期:2021-12-05
发布日期:2021-06-16
通讯作者:
黄骏
E-mail:jun.huang@sydney.edu.au
Received:2021-04-28
Online:2021-12-05
Published:2021-06-16
Contact:
Jun HUANG
E-mail:jun.huang@sydney.edu.au
Supported by:摘要:
固体酸是工业烃转化和生物质精炼中应用最广泛的非均相催化剂之一,了解它们的局部结构和酸性等性质有利于合理设计高效绿色固体酸催化剂,从而提高目标反应的活性和稳定性.近年来,固体核磁共振波谱在定性和定量表征固体酸的局部结构和酸性方面已显示出巨大的应用潜力,甚至可作为一种标准方法.二维固体核磁共振波谱的应用可以进一步揭示固体酸表面位点的结构对称性和不同位点的空间构效关系,从而加深对“催化剂结构-酸性-活性关系”的理解.在这篇综述中,我们总结了用于固体酸表征的固体核磁共振波谱方法和常规实验操作流程,并着重阐述了在使用和不使用探针分子的情况下,固体核磁共振波谱应用于固体酸局部结构和酸性性质研究的进展.
中图分类号:
杨文杰,黄骏. 基于固体核磁共振技术的固体酸结构、酸性及活性分析[J]. 波谱学杂志, 2021, 38(4): 460-473.
Wen-jie YANG,Jun HUANG. Analysis of Local Structure, Acidic Property and Activity of Solid Acids by Solid-State Nuclear Magnetic Resonance Spectroscopy[J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 460-473.
表1
固体酸催化剂中各种羟基的典型1H SSNMR化学位移(δH)
| Solid acid | Hydroxyl group | δH | Assignment | Reference |
| Zeolite | Si(OH) | 1.2~2.3 | 晶体表面或缺陷位的硅烷醇羟基 | [ |
| Al(OH) | 2.1~2.8 | 金属修饰分子筛上骨架外的桥接羟基 | [ | |
| Si(OH)Al | 3.3~4.4 | 超笼中桥接羟基 | [ | |
| Si(OH)Al | 4.6~8.0 | 方纳石笼中具有氢键或通过静电相互作用被骨架干扰的通道中桥接羟基 | [ | |
| Si(OH)Si | 10.0~16.0 | 探针分子吸附后的探测到的双硅烷醇基或以氢键相连的内部硅烷醇基 | [ | |
| Amorphous | Si(OH) | 1.7~2.3 | 骨架上硅烷醇基 | [ |
| Silica-alumina | Si(OH)Al/Al(OH) | 2.5~4.0 | 酸性羟基 | [ |
| Heteropoly acid | Keggin units H+ | 6.7~9.3 | 杂多酸的凯金(Keggin)结构中的酸性羟基 | [ |
| Metal-organic framework | Al(OH)Al | 1.7~2.7 | 骨架上桥接羟基 | [ |
| Mixed oxides | M(OH) | 2.0~6.7 | 末端金属羟基和桥连金属羟基 | [ |
| M(OH)..H2O..H2O | 9.5~10.3 | 通过氢键与水分子通过氢键相互作用两次的桥接金属羟基 | [ |
表2
部分常用探针分子以及基于化学位移变化的酸位特性判定
| Probe molecules | Strength of BAS | Strength of LAS | Reference |
| Deuterated acetonitrile (CD3CN) | δH: 3.6  Weak  | / | [ |
| Pyridine-d5 (C5D5N) | δH: 12  Strong  | / | [ |
| Acetone-2-13C ((CH3)213CO) | δC: 216  Weak  | δC: 233  Weak  | [ |
| 15N-pyridine | δN: 212  Strong  | δN: 260  Strong  | [ |
| Trimethylphosphine oxide (TMPO) | δP: 55  Weak  | δP: 37  Weak  | [ |
| Trimethylphosphine (TMP) | δP: -2  Larger J-coupling constant between 31P and 1H  | δP: -13 ~ -52 δP depends on Lewis centers, for identical Lewis centers, smaller δP  | [ |
表3
用于固体酸酸性特性表征的探针分子a
| Probe molecule | MAS NMR technique | Type | Strength | Density | Accessibility | |||||
| B | L | B | L | B | L | |||||
| Trimethylphosphine (TMP) | 31P | √ | √ | √ | √ | √ | √ | √ | ||
| Acetone-2-13C ((CH3)213CO) | 13C | √ | √ | √ | 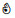 | ❌ | ❌ | 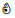 | ||
| Ammonia (NH3) | 1H | √ | √ | ❌ | ❌ | √ | √ | 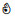 | ||
| Pyridine-d5 (C5D5N) | 1H | √ | ❌ | √ | ❌ |  | ❌ | √ | ||
| 15N-pyridine | 15N | √ | √ | √ | √ | √ | √ | √ | ||
| Trialkylphosphine oxides (R3PO) | 31P | √ | √ | √ |  | √ | √ | √ | ||
| Deuterated acetonitrile (CD3CN) | 1H | √ | ❌ | √ | ❌ | √ | ❌ | 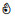 | ||
| Perfluorotributylamine | 1H | √ | ❌ | ❌ | ❌ |  | ❌ | √ | ||
| 1 | TANABE K. Solid acids and bases: their catalytic properties[M]. Elsevier, 2012. |
| 2 |
HUANG J , JIANG Y , MARTHALA V R , et al. Regioselective H/D exchange at the Side-Chain of ethylbenzene on dealuminated zeolite H-Y studied by in situ MAS NMR-UV/Vis spectroscopy[J]. Chem Phys Chem, 2008, 9 (8): 1107- 1109.
doi: 10.1002/cphc.200800065 |
| 3 |
YU Z , LI S , WANG Q , et al. Brønsted/lewis acid synergy in H-ZSM-5 and H-MOR zeolites studied by 1H and 27Al DQ-MAS solid-state NMR spectroscopy[J]. J Phys Chem C, 2011, 115 (45): 22320- 22327.
doi: 10.1021/jp203923z |
| 4 | ASTRUC D. Heterogeneous catalysis[M]. Organomet Chem Catal, 2007, 457-486. |
| 5 |
GORTE R J , CROSSLEY S P . A perspective on catalysis in solid acids[J]. J Catal, 2019, 375, 524- 530.
doi: 10.1016/j.jcat.2019.07.015 |
| 6 | WANG R D , XU B B , SONG Y H , et al. Methanol-water interaction in photocatalytic methanol reforming─An operando NMR study[J]. Chinese J Magn Reson, 2021, 38 (1): 43- 57. |
| 王睿迪, 徐贝贝, 宋艳红, 等. 原位核磁共振技术研究光催化甲醇重整过程中甲醇与水的相互作用[J]. 波谱学杂志, 2021, 38 (1): 43- 57. | |
| 7 |
WU X Q , XU S T , ZHANG W N , et al. Direct mechanism of the first carbon-carbon bond formation in the methanol-to-hydrocarbons process[J]. Angew Chem Int Ed, 2017, 129 (31): 9167- 9171.
doi: 10.1002/ange.201703902 |
| 8 | YE M , YANG Y N , ZHANG R , et al. Effects of co-catalysts and wavelength of light on the products of photocatalytic methanol reforming: An operando NMR study[J]. Chinese J Magn Reson, 2019, 36 (4): 490- 501. |
| 叶曼, 杨以宁, 张燃, 等. 原位核磁共振技术研究共催化剂类型以及光照波长对甲醇光催化重整产物的影响[J]. 波谱学杂志, 2019, 36 (4): 490- 501. | |
| 9 |
CHEN Y X , GONG K , JIAO F , et al. C-C bond formation in syngas conversion over zinc sites grafted on ZSM-5 Zeolite[J]. Angew Chem Int Ed, 2020, 59 (16): 6529- 6534.
doi: 10.1002/anie.201912869 |
| 10 |
XU S T , ZHANG W P , LIU X C , et al. Enhanced in situ continuous-flow MAS NMR for reaction kinetics in the nanocages[J]. J Am Chem Soc, 2009, 131 (38): 13722- 13727.
doi: 10.1021/ja904304h |
| 11 | WANG J X , FENG J W , CHEN J F , et al. Design and fabrication of a magic-angle spinning rotor for solid-state nuclear magnetic resonance probe[J]. Chinese J Magn Reson, 2019, 36 (4): 446- 455. |
| 王佳鑫, 冯继文, 陈俊飞, 等. 魔角旋转固体核磁共振探头中转子的研制[J]. 波谱学杂志, 2019, 36 (4): 446- 455. | |
| 12 | KEELER J. Understanding NMR spectroscopy[M]. John Wiley & Sons, 2011. |
| 13 |
LOWE I . Free induction decays of rotating solids[J]. Phys Rev Lett, 1959, 2 (7): 285.
doi: 10.1103/PhysRevLett.2.285 |
| 14 | HUNGER M. Solid-state NMR spectroscopy[M].//(CHESTER A W, DEROUANE E G, Eds.), Zeolite Characterization and Catalysis: A Tutorial, Springer Netherlands: Dordrecht, 2009, 65-105. |
| 15 | LIANG L X , DENG F , HOU G J . Quantitative cross polarization magic-angle spinning NMR spectroscopy in solids[J]. Chinese J Magn Reson, 2020, 37 (1): 1- 15. |
| 梁力鑫, 邓风, 侯广进. 固体核磁共振魔角旋转条件下的定量交叉极化技术(英文)[J]. 波谱学杂志, 2020, 37 (1): 1- 15. | |
| 16 |
HOU G J , DING S W , ZHANG L M , et al. Breaking the T1 constraint for quantitative measurement in magic angle spinning solid-state NMR spectroscopy[J]. J Am Chem Soc, 2010, 132 (16): 5538- 5539.
doi: 10.1021/ja909550f |
| 17 |
LI S H , ZHENG A M , SU Y C , et al. Extra-framework aluminium species in hydrated faujasite zeolite as investigated by two-dimensional solid-state NMR spectroscopy and theoretical calculations[J]. Phys Chem Chem Phys, 2010, 12 (15): 3895- 3903.
doi: 10.1039/b915401a |
| 18 |
LUAN Z H , CHENG C F , HE H Y , et al. Thermal stability of structural aluminum in the mesoporous molecular sieve MCM-41[J]. J Phys Chem, 1995, 99 (26): 10590- 10593.
doi: 10.1021/j100026a023 |
| 19 |
KENNEDY G J , AFEWORKI M , CALABRO D C , et al. 1H MAS NMR (magic-angle spinning nuclear magnetic resonance) techniques for the quantitative determination of hydrogen types in solid catalysts and supports[J]. Appl Spectrosc, 2004, 58 (6): 698- 704.
doi: 10.1366/000370204873105 |
| 20 |
WANG Z C , JIANG Y J , JIN F Z , et al. Strongly enhanced acidity and activity of amorphous silica-alumina by formation of pentacoordinated AlV species[J]. J Catal, 2019, 372, 1- 7.
doi: 10.1016/j.jcat.2019.02.007 |
| 21 |
WANG Z C , LI T , JIANG Y J , et al. Acidity enhancement through synergy of penta-and tetra-coordinated aluminum species in amorphous silica networks[J]. Nat Commun, 2020, 11, 225.
doi: 10.1038/s41467-019-13907-7 |
| 22 | ZHENG A, DENG F, LIU S. Acidity characterization of solid acid catalysts by solid-state 31P NMR of adsorbed phosphorus-containing probe molecules[M].//WEBB G A. Annu Rep NMR Spectrosc. APNMR, Academic Press. 2014, 47-108. |
| 23 |
LI S H , LAFON O , WANG W Y , et al. Recent advances of solid-state NMR spectroscopy for microporous materials[J]. Adv Mater, 2020, 32 (44): 2002879.
doi: 10.1002/adma.202002879 |
| 24 |
LI S H , ZHOU L , ZHENG A M , et al. Recent advances in solid state NMR characterization of zeolites[J]. Chinese J Catal, 2015, 36 (6): 789- 796.
doi: 10.1016/S1872-2067(14)60290-4 |
| 25 |
GABRIENKO A A , DANILOVA I G , ARZUMANOV S S , et al. Strong acidity of silanol groups of zeolite beta: Evidence from the studies by IR spectroscopy of adsorbed CO and 1H MAS NMR[J]. Microporous Mesoporous Mater, 2010, 131 (1-3): 210- 216.
doi: 10.1016/j.micromeso.2009.12.025 |
| 26 |
BORONAT M , CORMA A . Factors controlling the acidity of zeolites[J]. Catal Lett, 2015, 145 (1): 162- 172.
doi: 10.1007/s10562-014-1438-7 |
| 27 |
PAZE C , ZECCHINA A , SPERA S , et al. Comparative IR and 1H-MAS NMR study of adsorption of CD3CN on zeolite H-β: evidence of the presence of two families of bridged Bronsted sites[J]. Phys Chem Chem Phys, 1999, 1 (10): 2627- 2629.
doi: 10.1039/a902621e |
| 28 |
BORONAT M , CORMA A . Factors controlling the acidity of zeolites[J]. Catal Lett, 2015, 145 (1): 162- 172.
doi: 10.1007/s10562-014-1438-7 |
| 29 |
BARAN R , MILLOT Y , ONFROY T , et al. Influence of the nitric acid treatment on Al removal, framework composition and acidity of BEA zeolite investigated by XRD, FTIR and NMR[J]. Microporous Mesoporous Mater, 2012, 163, 122- 130.
doi: 10.1016/j.micromeso.2012.06.055 |
| 30 | GAO X Z , ZHANG Y , WANG X M , et al. Structure and Acidity changes in ultra-stable Y zeolites during hydrothermal aging: A solid-state NMR spectroscopy study[J]. Chinese J Magn Reson, 2020, 37 (1): 95- 103. |
| 高秀枝, 张翊, 王秀梅, 等. NMR研究超稳Y分子筛水热老化过程中结构与酸性的变化[J]. 波谱学杂志, 2020, 37 (1): 95- 103. | |
| 31 |
GABRIENKO A , DANILOVA I G , ARZUMANOV S S , et al. Direct measurement of zeolite Brønsted acidity by FTIR spectroscopy: Solid-state 1H MAS NMR approach for reliable determination of the integrated molar absorption coefficients[J]. J Phys Chem C, 2018, 122 (44): 25386- 25395.
doi: 10.1021/acs.jpcc.8b07429 |
| 32 |
CHEN K Z , ABDOLRHAMANI M , SHEETS E , et al. Direct detection of multiple acidic proton sites in zeolite HZSM-5[J]. J Am Chem Soc, 2017, 139 (51): 18698- 18704.
doi: 10.1021/jacs.7b10940 |
| 33 |
ABDOLRAHMANI M , CHEN K , WHITE J . Assessment, control, and impact of brønsted acid site heterogeneity in zeolite HZSM-5[J]. J Phys Chem C, 2018, 122 (27): 15520- 15528.
doi: 10.1021/acs.jpcc.8b04283 |
| 34 | XU J, WANG Q, LI S H, et al. Solid-state NMR characterization of acid properties of zeolites and solid acid catalysts[M].//Solid-state NMR in zeolite catalysis. Springer, 2019, 159-197. |
| 35 |
HAIJJAR R , MILLOT Y , MAN P P , et al. Two kinds of framework Al sites studied in BEA zeolite by X-ray diffraction, Fourier transform infrared spectroscopy, NMR techniques, and V probe[J]. J Phys Chem C, 2008, 112 (51): 20167- 20175.
doi: 10.1021/jp808356q |
| 36 | ESCRIBANO V S , GARBARINO G , FINOCCHIO E , et al. γ-Alumina and amorphous silica-alumina: structural features, acid sites and the role of adsorbed water[J]. TOP CATAL, 2017, 60 (19, 20): 1554- 1564. |
| 37 |
CREPEAU G , MONTOUILLOUT V , VIMONT A , et al. Nature, structure and strength of the acidic sites of amorphous silica alumina: an IR and NMR study[J]. J Phys Chem B, 2006, 110 (31): 15172- 15185.
doi: 10.1021/jp062252d |
| 38 |
BRUNNER E . Characterization of solid acids by spectroscopy[J]. Catal Today, 1997, 38 (3): 361- 376.
doi: 10.1016/S0920-5861(97)81504-9 |
| 39 |
DEC S F , HERRING A M . Solid-state 1H NMR study of structures and dynamics of proton sites in group Ⅱ salts of 12-tungstophosphoric acid and related compounds[J]. J Phys Chem C, 2018, 122 (27): 15539- 15557.
doi: 10.1021/acs.jpcc.8b04370 |
| 40 | HUANG J , JIANG Y J , REDDG MARTHALA V A , et al. In situ 1H MAS NMR investigations of the H/D exchange of alkylaromatic hydrocarbons on zeolites HY, La, Na-Y, and H-ZSM-5[J]. Microporous Mesoporous Mater, 2007, 99 (1, 2): 86- 90. |
| 41 | RIEMER T , SPIELBAUER D , HUNGER M , et al. Superacid properties of sulfated zirconia as measured by Raman and 1H MAS NMR spectroscopy[J]. J Chem Soc Chem Comm, 1994, 10, 1181- 1182. |
| 42 |
GUN'KO V M , ZARKO V V , TUROV V V , et al. Characterization of fumed alumina/silica/titania in the gas phase and in aqueous suspension[J]. J Colloid Interface Sci, 1999, 220 (2): 302- 323.
doi: 10.1006/jcis.1999.6481 |
| 43 |
BORGES L D , DE MACEDO J . Solid-state dealumination of zeolite Y: Structural characterization and acidity analysis by calorimetric measurements[J]. Microporous Mesoporous Mater, 2016, 236, 85- 93.
doi: 10.1016/j.micromeso.2016.08.031 |
| 44 |
JIANG Y J , HUANG J , DAI W L , et al. Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts[J]. Solid State Nucl Magn Reson, 2011, 39 (3-4): 116- 141.
doi: 10.1016/j.ssnmr.2011.03.007 |
| 45 |
WANG Z C , JIANG Y J , LAFON O , et al. Brønsted acid sites based on penta-coordinated aluminum species[J]. Nat Commun, 2016, 7, 13820.
doi: 10.1038/ncomms13820 |
| 46 |
LI S H , LI J , ZHENG A M , et al. Solid-state NMR characterization of the structure and catalytic reaction mechanism of solid acid catalysts[J]. ACTA PHYS-CHIM SIN, 2017, 33 (2): 270- 282.
doi: 10.3866/PKU.WHXB201611022 |
| 47 |
YANG W J , WANG Z C , HUANG J , et al. Qualitative and quantitative analysis of acid properties for solid acids by solid-state nuclear magnetic resonance spectroscopy[J]. J Phys Chem C, 2021, 125 (19): 10179- 10197.
doi: 10.1021/acs.jpcc.1c01887 |
| 48 |
SONG C H , CHU Y Y , WANG M , et al. Cooperativity of adjacent Brønsted acid sites in MFI zeolite channel leads to enhanced polarization and cracking of alkanes[J]. J Catal, 2017, 349, 163- 174.
doi: 10.1016/j.jcat.2016.12.024 |
| 49 |
PERRAS F A , WANG Z C , KOBAYASHI T , et al. Shedding light on the atomic-scale structure of amorphous silica-alumina and its Brønsted acid sites[J]. Phys Chem Chem Phys, 2019, 21 (35): 19529- 19537.
doi: 10.1039/C9CP04099D |
| 50 |
GAO P , WANG Q , XU J , et al. Brønsted/Lewis acid synergy in methanol-to-aromatics conversion on Ga-modified ZSM-5 zeolites, as studied by solid-state NMR spectroscopy[J]. ACS Catal, 2018, 8 (1): 69- 74.
doi: 10.1021/acscatal.7b03211 |
| 51 |
PENG L M , GREY C P . Diphosphine probe molecules and solid-state NMR investigations of proximity between acidic sites in zeolite HY[J]. Microporous Mesoporous Mater, 2008, 116 (1-3): 277- 283.
doi: 10.1016/j.micromeso.2008.04.014 |
| 52 |
CHEN K Z , HORSTMEIER S , NGUYEN V T , et al. Structure and catalytic characterization of a second framework Al(IV) site in zeolite catalysts revealed by NMR at 35.2 T[J]. J Am Chem Soc, 2020, 142 (16): 7514- 7523.
doi: 10.1021/jacs.0c00590 |
| 53 |
PENG L M , LIU Y , KIM N . Detection of Brønsted acid sites in zeolite HY with high-field 17O-MAS-NMR techniques[J]. Nat Mater, 2005, 4 (3): 216- 219.
doi: 10.1038/nmat1332 |
| 54 |
PENG L M , HUO H , GAN Z H . 17O MQMAS NMR studies of zeolite HY[J]. Microporous Mesoporous Mater, 2008, 109 (1-3): 156- 162.
doi: 10.1016/j.micromeso.2007.04.039 |
| 55 |
PENG L M , HUO H , LIU Y , et al. 17O magic angle spinning NMR studies of Brønsted acid sites in zeolites HY and HZSM-5[J]. J Am Chem Soc, 2007, 129 (2): 335- 346.
doi: 10.1021/ja064922z |
| 56 |
SHEN L , PENG L M . 17O solid-state NMR studies of oxygen-containing catalysts[J]. Chinese J Catal, 2015, 36 (9): 1494- 1504.
doi: 10.1016/S1872-2067(15)60931-7 |
| 57 | SHEN L , WU X P , WANG Y , et al. 17O solid-state NMR studies of ZrO2 nanoparticles[J]. J Phys Chem C, 2019, 12 (7): 4158- 4167. |
| 58 | SHEN L , WANG Y , DU J H , et al. Interactions of γ-alumina with water via multinuclear solid-state NMR spectroscopy[J]. Chem Cat Chem, 2020, 12 (6): 1569- 1574. |
| 59 |
SUN T T , XU S T , XIAO D , et al. Water-induced structural dynamic process in molecular sieves under mild hydrothermal conditions: Ship-in-a-bottle strategy for acidity identification and catalyst modification[J]. Angew Chem Int Ed, 2020, 132 (46): 20853- 20862.
doi: 10.1002/ange.202009648 |
| 60 |
GUNTHER W R , MICHAELIS V K , CAPORINI M A , et al. Dynamic nuclear polarization NMR enables the analysis of Sn-Beta zeolite prepared with natural abundance 119Sn precursors[J]. J Am Chem Soc, 2014, 136 (17): 6219- 6222.
doi: 10.1021/ja502113d |
| 61 |
ZHENG A M , LIU S B , DENG F . 31P NMR chemical shifts of phosphorus probes as reliable and practical acidity scales for solid and liquid catalysts[J]. Chem Rev, 2017, 117 (19): 12475- 12531.
doi: 10.1021/acs.chemrev.7b00289 |
| 62 |
WANG Y X , XIN S H , CHU Y Y , et al. Influence of trimethylphosphine oxide loading on the measurement of zeolite acidity by solid-state NMR spectroscopy[J]. J Phys Chem C, 2021, 125 (17): 9497- 9506.
doi: 10.1021/acs.jpcc.1c01789 |
| 63 | XU J , DENG F . Solid-state NMR in interdisciplinary research[J]. Acta Phys Chim Sin, 2020, 36 (4): 1912074- 0. |
| 徐君, 邓风. 多学科交叉研究中的固体核磁共振[J]. 物理化学学报, 2020, 36 (4): 1912074- 0. | |
| 64 |
ZHENG A M , HUANG S J , LIU S B , et al. Acid properties of solid acid catalysts characterized by solid-state 31P NMR of adsorbed phosphorous probe molecules[J]. Phys Chem Chem Phys, 2011, 13 (33): 14889- 14901.
doi: 10.1039/c1cp20417c |
| 65 |
ZHENG A M , ZHANG H L , LU X , et al. Theoretical predictions of 31P NMR chemical shift threshold of trimethylphosphine oxide absorbed on solid acid catalysts[J]. J Phys Chem B, 2008, 112 (15): 4496- 4505.
doi: 10.1021/jp709739v |
| 66 |
HUANG J , JIANG Y J , REDDY MARTHALA V R , et al. Concentration and acid strength of hydroxyl groups in zeolites La, Na-X and La, Na-Y with different lanthanum exchange degrees studied by solid-state NMR spectroscopy[J]. Microporous Mesoporous Mater, 2007, 104 (1-3): 129- 136.
doi: 10.1016/j.micromeso.2007.01.016 |
| 67 |
GONG K , LIU Z M , LIANG L X , et al. Acidity and local confinement effect in mordenite probed by solid-state NMR spectroscopy[J]. J Phys Chem Lett, 2021, 12 (9): 2413- 2422.
doi: 10.1021/acs.jpclett.0c03610 |
| 68 |
LUO Q , DENG F , YUAN Z Y , et al. Using trimethylphosphine as a probe molecule to study the acid sites in Al-MCM-41 materials by solid-state NMR spectroscopy[J]. J Phys Chem B, 2003, 107 (11): 2435- 2442.
doi: 10.1021/jp0213093 |
| 69 | ENGELHARDT G. Solid state NMR spectroscopy applied to zeolites[M].//Studies in surface science and catalysis, Elsevier: 2001, 137, 387-418. |
| 70 | HUANG J , JIANG Y J , REDDY MARTHALA V R , et al. Characterization and acidic properties of aluminum-exchanged zeolites X and Y[J]. J Phys Chem B, 2008, 112 (10): 3811- 3818. |
| 71 |
HAW J F , NICHOLAS J B , XU T , et al. Physical organic chemistry of solid acids: lessons from in situ NMR and theoretical chemistry[J]. Acc Chem Res, 1996, 29 (6): 259- 267.
doi: 10.1021/ar950105k |
| 72 |
SU X , XU S T , TIAN P , et al. Investigation of the strong Brønsted acidity in a novel SAPO-type molecular sieve, DNL-6[J]. J Phys Chem C, 2015, 119 (5): 2589- 2596.
doi: 10.1021/jp511670q |
| 73 |
GUNTHER W R , MICHAELIS V K , GRIFFIN R C , et al. Interrogating the Lewis acidity of metal sites in beta zeolites with 15N pyridine adsorption coupled with MAS NMR spectroscopy[J]. J Phys Chem C, 2016, 120 (50): 28533- 28544.
doi: 10.1021/acs.jpcc.6b07811 |
| 74 |
CHU Y Y , YU Z W , ZHENG A M , et al. Acidic strengths of Brønsted and Lewis acid sites in solid acids scaled by 31P NMR chemical shifts of adsorbed trimethylphosphine[J]. J Phys Chem C, 2011, 115 (15): 7660- 7667.
doi: 10.1021/jp200811b |
| 75 |
YI X F , DING L H , LI G C , et al. Insights into the reaction mechanism of propene H/D exchange over acidic zeolite catalysts from theoretical calculations[J]. Catal Sci Technol, 2016, 6 (16): 6328- 6338.
doi: 10.1039/C6CY00757K |
| 76 |
KUBOTA T , OSUGA R , YOKOI T , et al. Consideration of acid strength of a single oh group on zeolites by isotope exchange reaction with ethane at high temperatures[J]. TOP CATAL, 2017, 60 (19-20): 1496- 1505.
doi: 10.1007/s11244-017-0834-9 |
| 77 |
SOMMER J , HABERMACHER D , JOST R , et al. Activation of small alkanes on solid acids. An H/D exchange study by liquid and solid-state NMR: the activation energy and the inhibiting effect of carbon monoxide[J]. J Catal, 1999, 181 (2): 265- 270.
doi: 10.1006/jcat.1998.2302 |
| 78 |
KAZANSKY V B . Adsorbed carbocations as transition states in heterogeneous acid catalyzed transformations of hydrocarbons[J]. Catal Today, 1999, 51 (3-4): 419- 434.
doi: 10.1016/S0920-5861(99)00031-0 |
| [1] | 夏锡锋,张文静,林芝晔,柯晓康,温玉洁,王芳,陈俊超,彭路明. 氧化物纳米材料表面结构与性质的固体核磁共振波谱研究[J]. 波谱学杂志, 2021, 38(4): 533-542. |
| [2] | 王子春,黄骏,姜怡娇. 五配位铝强化硅铝固体酸的固体核磁共振研究[J]. 波谱学杂志, 2021, 38(4): 552-570. |
| [3] | 喻志武, 郑安民, 王强, 黃信炅, 邓风, 刘尚斌. 固体核磁共振研究固体酸催化剂酸性进展[J]. 波谱学杂志, 2010, 27(4): 485-515. |
| [4] | Seen Ae CHAE, Young Eun LEE, Oc Hee HAN, Sang Geul LEE. 用多核固体核磁共振研究CsH2PO4 和CsH5(PO4)2的制备[J]. 波谱学杂志, 2010, 27(3): 436-444. |
| [5] | 作者:徐君 导师:邓风. 固体酸催化剂及分子筛晶化过程的核磁共振研究[J]. 波谱学杂志, 2007, 24(3): 368-370. |
| [6] | 作者:郑安民 导师:邓风. 量化计算在预测NMR参数和固体催化剂酸性中的应用[J]. 波谱学杂志, 2006, 23(4): 543-544. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 222
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 185
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
