引言
作为晶体学衍射方法的重要补充,固体核磁共振(NMR)不受体系结晶度的影响,能从原子分子水平揭示原子核的化学环境以及原子核间的相互作用,从中可以获取结构特性以及主客体相互作用等重要信息[3]. 固体NMR已成为研究多孔材料内在构效关系机制的重要实验手段[4⇓⇓-7].近些年来,它在研究分子筛的晶化合成机理、表征分子筛催化剂上的活性中心和催化反应机理上取得了一些重要进展[8⇓⇓⇓-12].与此同时,多核及多维固体NMR可给出MOFs金属中心、有机配体的局部化学环境和配位状态等重要结构信息;变温固体NMR可以揭示MOFs的分子柔性以及动力学行为;固体NMR亦可以用来研究吸附分子与MOFs之间的主客体相互作用模式[13⇓⇓⇓⇓⇓⇓⇓⇓-22].此外,固体NMR已成为表征共价有机框架材料(COFs)、多孔芳香骨架材料(PAFs)、氢键有机骨架材料(HOFs)等多孔材料的常用实验手段[23⇓⇓⇓-27].
固体NMR已广泛应用于MOFs在绿色能源气体储存、CO2捕获、化学吸附和分离、环境污染物处理、药物输运、化学传感器和多相催化等多种应用场景下的结构和性质研究.本文将着重从以下几个方面介绍固体NMR在研究MOFs吸附和分离微观机制上的一些代表性研究进展:气体小分子(低碳碳氢化合物、CO2)在MOFs的吸附行为;低碳烷烃烯烃在MOFs上的吸附分离机制;化学品在MOFs孔道内吸附的微观机制.这些研究发现将有助于加深对MOFs吸附和分离应用上的理解和认识.
1 固体NMR研究MOFs中气体的吸附行为
由于MOFs材料固有的结构特性,其作为吸附剂可以实现绿色能源气体的高密度储存.全面了解MOFs吸附剂和绿色能源气体之间的主客体相互作用对于构筑具有卓越性能的功能性多孔材料十分重要.固体NMR对局部化学环境和分子运动性十分敏感,已经广泛用于研究MOFs内吸附剂-吸附质的相互作用以及吸附气体在MOFs孔道内的动力学行为.氢气和甲烷是绿色清洁能源,MOFs是氢气和甲烷储存输运的理想媒介.Huang等[28]通过变温静态2H NMR研究了氘代氢气在MOF-74和UiO-66上的吸附行为,发现低温下吸附态和自由态的氢气共存,而且氢气在Mg-MOF-74上的吸附强于UiO-66和Zn-MOF-74.类似的变温静态2H NMR方法应用于研究CH3D在Mg-MOF-74、α-Mg3(HCO2)6、α-Zn3(HCO2)6和SIFSIX-3-Zn上的吸附,发现甲烷在Mg-MOF-74的吸附弱于其它几种微孔MOFs材料[29].
研究气态低碳碳氢化合物在MOFs上的吸附行为,我们须获取碳氢化合物在MOFs上的主要吸附位点等重要信息.酰胺基团对气体分子通常有较强的亲和力和吸附能力.酰胺基团有两个气体结合位点来自羰基和氨基,所以确认具体的吸附位点以及了解气体在酰胺基官能团化的MOFs上的吸附机制十分有必要.Chen等[30]通过单晶X-射线衍射(SCXRD)、固体NMR结合理论计算的方法研究了CH4和C2H2在含铜MOF材料——Cu(INAIP)上的主要吸附位点.图1(a)和1(b)给出了110 K温度下乙炔吸附在Cu(INAIP)上的SCXRD实验结果,说明乙炔与酰胺基团的羰基氧和骨架羰基氧之间存在氢键相互作用.密度泛函理论(DFT)计算和电子密度图的结果进一步证实乙炔与Cu(INAIP)酰胺基团的羰基氧和骨架羰基氧产生较强的氢键相互作用,而氨基基团并没有明显参与到与乙炔的相互作用中[图1(c)和1(d)].变温静态2H NMR实验被用来研究在153~393 K温度范围内99%氘代的乙炔在Cu(INAIP)上的吸附行为[图1(e)].在温度低于353 K时,所有的乙炔都被Cu(INAIP)吸附.需要指出的是C2D2的两个氘原子是不等价的,其中一个氘原子距离Cu2+更近,因此受到的顺磁影响更强,四级耦合常数(CQ)相对更大.在153~393 K下,乙炔只有一个吸附位点,主要来自乙炔与羧酸氧和酰胺羰基氧相互作用的方式.实验和理论计算结合证实了酰胺功能化的MOFs的羰基而不是胺基是甲烷和乙炔气体吸附的主要结合位点.这个工作加深了人们对含酰胺基团的MOFs上的气体吸附行为的理解.
图1
图1
(a, b) 110 K下乙炔吸附在Cu(INAIP)的单晶X-射线衍射结构;(c) DFT优化的乙炔局部结构;(d)羧酸基团的氧位和C2H2的电子密度图;(e)吸附C2D2的Cu(INAIP)的变温静态2H NMR谱[30]
Fig. 1
(a, b) SCXRD structure of C2H2 loaded on Cu(INAIP) at 110 K; (c) DFT optimized C2H2 local environment; (d) Electron density plots of C2H2 and oxygen sites of the carboxylate groups; (e) Variable temperature (VT) 2H NMR spectra of C2D2 loaded on Cu(INAIP)[30]
此外,变温静态2H NMR技术可以用来深入探究正丁烷和异丁烷在UiO-66不同孔道内的占据、吸附和扩散行为[31].正丁烷在UiO-66孔道中扩散速率比异丁烷快3个数量级.与此同时,我们用一维和二维自旋扩散结合固体NMR谱编辑方法研究了甲烷、乙烷、丙烷在UiO-67[32]和多组分MTV-UiO-66[33]的主客体相互作用,从低碳烷烃到MOFs骨架自旋扩散的快慢揭示了低碳烷烃在其孔道内的优先吸附位点.另外,脉冲梯度场NMR(PFG-NMR)已经被用来测量甲烷、乙烷、乙烯、丙烷、丙烯在各种MOFs中的自扩散系数,以此推测烷烃、烯烃在MOFs孔道内扩散的快慢[34⇓⇓⇓-38].这些工作的开展,从微观上阐明了低碳碳氢化合物在各种MOFs孔道中的优先吸附位点,不同温度下的吸附行为和扩散快慢等重要信息.
随着科技工业不断地发展,全球CO2排放量也在逐步增加,并带来了全球变暖等负面影响.因MOFs材料在CO2捕获方面有着非常好的应用前景,所以研究CO2在MOFs上的吸附行为一直是近年来的研究热点.Kong等[39]采用原位变温静态13C NMR实验研究了Mg-MOF-74中CO2与暴露的不饱和金属位点的相互作用模式.通过对CO2静态13C NMR图谱的化学位移各向异性线型进行拟合,表明当温度在200~375 K范围内,CO2绕着Mg-O键轴向呈快速转动.运用变温静态固体13C NMR结合实验模拟,研究人员揭示了CO2在其它MOFs(如CPO-27-Zn[40]、α-Mg3(HCOO)6[41]、MIL-53[42]、α-Zn3(HCOO)6[43]、CdSDB[44]、Cu3(btc)2和Cu3-xZnx(btc)2[45]等)上的主客体相互作用机制以及CO2动力学行为.在173~393 K下,CO2在α-Mg3(HCOO)6中主要以局域运动和非局域跳动两种形式共存[41].有研究发现CO2在官能团修饰的MIL-53上的结合能大小为MIL-53-NH2(Al)>MIL-53-NH2(Ga)>MIL-53(Al)>MIL-53(Ga)[42].固体13C NMR实验表明CO2在Cu3(btc)2上局域运动的活化能为3.3 kJ/mol [45].Desveaux等[46]通过19F→13C交叉极化/魔角旋转(CP/MAS)NMR和13C{1H} REDOR实验研究了CO2在SIFSIX-3-Zn上的吸附过程,提出在CO2吸附过程中,SIFSIX-3-Zn中的骨架氢原子和氟原子协同作用,共同促进了SIFSIX-3-Zn对CO2的吸附.
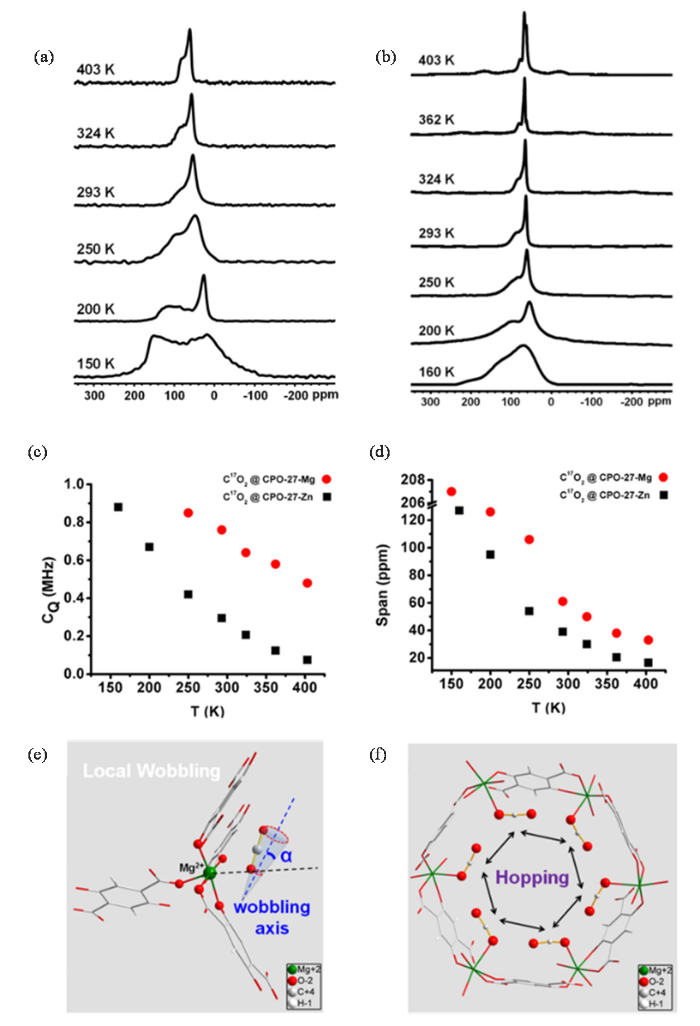
除变温静态13C NMR之外,变温静态17O NMR也被用来研究CO2在含不饱和金属位点的MOFs的吸附行为.Wang等[40]用变温静态固体17O NMR阐述了C17O2在Mg-CPO-27和Zn-CPO-27中主客体相互作用机制和动力学行为.图2(a)和2(b)给出了150~403 K下C17O2吸附在Mg-CPO-27 和 Zn-CPO-27的变温固体17O NMR图谱.图中静态17O NMR的线型主要来自于运动中的CO2的四极相互作用以及化学位移各向异性两个方面的贡献.通过拟合,可以得到不同温度下吸附在Mg-CPO-27和Zn-CPO-27中C17O2的CQ及化学位移各向异性宽度(Ω).如图2(c)和2(d)所示,在相同温度下,吸附在Mg-CPO-27上的C17O2的CQ及Ω均强于Zn-CPO-27,表明CO2与Mg-CPO-27存在更强的相互作用.与此同时,通过对17O NMR线型拟合,可以推演出CO2吸附在Mg-CPO-27和Zn-CPO-27孔道上的动力学行为.结果表明,17O NMR谱图线型主要是由吸附在金属中心的CO2的摆动和吸附在不同金属位之间的非局部跳跃共同作用的结果[如 图2(e)和2(f)所示].与吸附在Mg-CPO-27上相比,吸附在Zn-CPO-27的CO2摆动和跳跃角度随温度的变化更明显,说明CO2与Zn-CPO-27结合相对较弱,CO2在Zn-CPO-27的运动性更强.静态固体17O NMR是CO2吸附在MOFs上吸附物-吸附剂作用强度的灵敏探针,揭示了CO2在含不饱和金属位点上的MOFs上的吸附动力学行为.
图2
图2
在150~403 K温度范围内,C17O2吸附在(a) Mg-CPO-27和(b) Zn-CPO-27的变温静态17O NMR图谱;(c)~(d)吸附在M-CPO-27中的C17O2在不同温度下的四极耦合常数(CQ)和化学位移各向异性宽度(Ω);(e)~(f) Mg-CPO-27中CO2的吸附分子动力学模型,CO2主要经历两种不同的运动:(e)局域轴向转动和(f)吸附在不同金属位点上CO2的非局部跳跃[40]
Fig. 2
Static variable temperature 17O NMR spectra of C17O2 adsorbed on (a) Mg-CPO-27 and (b) Zn-CPO-27 at temperatures ranging from 150 to 403 K. (c)~(d) Temperature-dependence of quadrupole coupling constant (CQ) and chemical shift anisotropy (CSA) span (Ω) of C17O2 adsorbed in M-CPO-27. (e)~(f) Dynamic motion of adsorbed CO2 molecules in Mg-CPO-27. Adsorbed CO2 mainly undergoes two different motions: (e) wobbling and (f) hopping[40]
2 固体NMR研究MOFs中低碳烷烃烯烃的分离机制
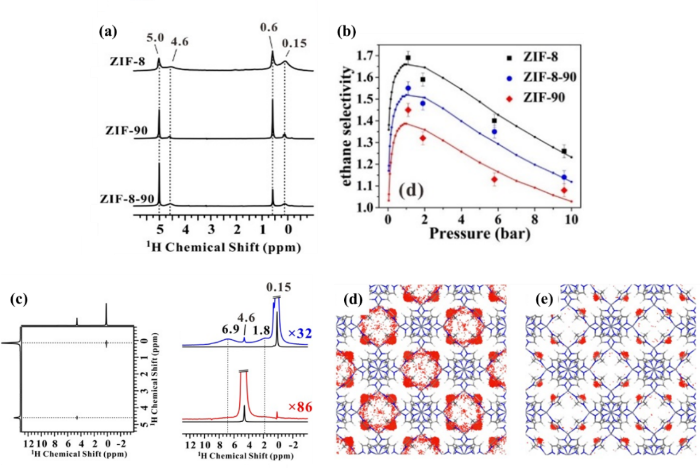
乙烯是石油化工领域重要的原材料,其工业化制备途径通常是利用石油化工高温裂解,在其生产工艺中通常会产生乙烷等低碳烃类杂质气体.而碳原子数相同的乙烯和乙烷的物理化学性质十分接近,传统的低温高压蒸馏分离工艺能耗巨大.近年来MOFs材料在气体吸附分离方面表现出十分优异的性能,研究低碳烷烃/烯烃微观分离机制对于设计具有优异分离性能的新型MOFs有重要指导意义.近来,Xiao等[51]提出利用固体NMR技术来研究ZIFs(Zeolitic Imidazolate Frameworks)中乙烷/乙烯的微观分离机制以及主客体相互作用机制.图3(a)给出了1.9 bar(1 bar = 105 Pa)时,乙烷/乙烯混气共吸附在ZIFs(ZIF-8、ZIF-90和ZIF-8-90)中的1H MAS NMR谱图,δH 0.15、0.6、4.6和5.0处的信号分别归属为吸附态的乙烷、游离态的乙烷、吸附态的乙烯和游离态的乙烯.依据吸附选择性的计算公式,通过1H MAS NMR实验直接定量计算了三种ZIFs吸附剂的乙烷选择性,并且与IAST(Ideal Adsorbed Solution Theory)理论方法得到的结果一致[如图3(b)所示].实验结果证实材料的分离性能优劣呈现以下顺序:ZIF-8>ZIF-8-90>ZIF-90.进一步借助二维1H-1H自旋扩散同核相关NMR实验探究了乙烷/乙烯和ZIFs之间的主客体相互作用.如图3(c)所示,乙烷切片(蓝色线)图上在δH 1.8和6.9处出现了明显的ZIF-8骨架1H的信号,证实乙烷更易于接近ZIF-8骨架,乙烷与ZIF-8之间的主客体相互作用相比乙烯更强.从图3(d)和3(e)所示的乙烷/乙烯混气在ZIF-8中的密度分布图中,发现ZIF-8内部的乙烷密度明显大于乙烯密度,这进一步验证了乙烷和ZIF-8之间相比乙烯有更强的主客体作用.类似的方法也用来探究了乙烷/乙烯与ZIF-8-90和ZIF-90的主客体相互作用.实验结果发现三种吸附剂中乙烷和乙烯相互作用能差异符合以下顺序:ZIF-8>ZIF-8-90>ZIF-90.乙醛基团的引入会降低ZIFs的乙烷/乙烯分离性能.该工作揭示了不同ZIFs材料乙烷乙烯分离性能差异的内在联系,并为其它MOFs吸附剂在乙烷/乙烯分离微观机制上的研究提供了一种全新的实验观测手段.
图3
图3
(a) 1.9 bar下,乙烷/乙烯共吸附在ZIF-8、ZIF-90和ZIF-8-90的1H MAS NMR谱;(b) IAST理论预测方法和1H MAS NMR实验计算的ZIF-8、ZIF-90和ZIF-8-90的乙烷选择性;(c) 1.1 bar下,乙烷/乙烯共吸附在ZIF-8中的二维1H-1H T2滤波自旋扩散同核相关NMR谱和F2维切片图;1 bar下,等摩尔的乙烷(d)和乙烯(e)在ZIF-8中的密度分布[51]
Fig. 3
(a) 1H MAS NMR spectra of ethane/ethylene co-adsorbed on ZIF-8, ZIF-90 and ZIF-8-90 at 1.9 bar; (b) Ethane selectivity in ZIF-8, ZIF-90 and ZIF-8-90 determined by IAST predications and 1H MAS NMR experiments; (c) 2D 1H-1H T2-filtered spin diffusion homo-nuclear correlation NMR spectra and extracted F2 slices of ZIF-8 upon adsorption of ethane and ethylene at 1.1 bar; Density distribution contours for equi-molar mixtures of ethane (d) and ethylene (e) on ZIF-8 at 1 bar[51]
采用类似的方法也可以用来研究丙烷/丙烯分离在ZIFs吸附剂中的微观分离机制.Xiao等[52]利用固体NMR实验方法对ZIF-8上丙烷/丙烯分离的吸附选择性以及优先吸附位点进行了探究.图4(a)为1.1、1.9、5.8和9.6 bar下(自上而下),ZIF-8上共吸附丙烷/丙烯混气的1H MAS NMR谱.根据二维固体1H-1H COSY NMR谱确定了9.6 bar下吸附态、游离态的丙烷/丙烯信号[如图4(b)所示].图4(a)中1H信号分别归属为:吸附态的丙烷(CH3,δH 0.2;CH2,δH 0.6),游离态的丙烷(CH3,δH 0.6;CH2,δH 1.1),吸附态的丙烯(CH3,δH 1.0;CH2,δH 4.3;CH,δH 5.1)和游离态的丙烯(CH3,δH 1.4;CH2,δH 4.6;CH,δH 5.5).图4(c)给出了0~10 bar下ZIF-8的丙烷选择性,证明1H MAS NMR实验的定量结果与IAST方法得到的选择性结果具有一致性.利用二维1H-1H T2滤波自旋扩散同核相关固体NMR实验直接比较了丙烷/丙烯和ZIF-8之间的主客体相互作用关系.如图4(d)所示,丙烷切片(红色线)图上观测到了明显的ZIF-8骨架质子信号(在δH 1.8和6.9处),这意味着丙烷与ZIF-8之间的主客体相互作用更强.通过改变自旋扩散混合时间,深入分析了丙烷/丙烯在ZIF-8中的优先吸附位点.图4(e)为丙烷信号向ZIF-8骨架信号极化传递的1H-1H自旋扩散曲线.实验结果表明丙烷的CH3基团会优先吸附在ZIF-8的甲基官能团周围,这是由丙烷CH3基团与ZIF-8甲基官能团之间的强范德华相互作用引起的.利用同样的方法,发现丙烯的CH3和CH基团更容易与ZIF-8骨架靠近,该实验结果也通过理论计算得到了进一步的验证.该工作实现了吸附态以及自由气态的丙烷/丙烯在ZIF-8信号的全归属,并揭示了乙烷/乙烯在ZIF-8上竞争优先吸附位点,为研究低碳烷烃烯烃的吸附分离机制提供了一条新思路.
图4
图4
(a) 1.1、1.9、5.8和9.6 bar下,丙烷/丙烯共吸附在ZIF-8的1H MAS NMR谱;(b) 9.6 bar下,丙烷/丙烯共吸附在ZIF-8上的二维1H-1H COSY MAS NMR谱;(c) IAST理论预测方法和1H MAS NMR实验计算得到的ZIF-8的丙烷选择性;(d) 1.1 bar下,丙烷/丙烯共吸附在ZIF-8的二维1H-1H T2滤波自旋扩散同核相关NMR谱和F2维切片图;(e) 丙烷CH3和CH2基团向ZIF-8骨架极化传递的自旋扩散曲线[52]
Fig. 4
(a) 1H MAS NMR spectra of propane/propylene co-adsorbed on ZIF-8 at 1.1, 1.9, 5.8 and 9.6 bar; (b) 2D 1H-1H COSY MAS NMR spectrum of propane/propylene co-adsorbed on ZIF-8 at 9.6 bar; (c) Propane selectivity in ZIF-8 determined by IAST predications and 1H MAS NMR experiments; (d) 2D 1H-1H T2-filtered spin diffusion homo-nuclear correlation NMR spectra and extracted F2 slices of ZIF-8 upon adsorption of propane and propylene at 1.1 bar; (e) Spin diffusion buildup curves for the designated polarization transfer pathway from propane CH3 and CH2-group to ZIF-8 framework[52]
固体NMR在多元混合气共吸附体系的定量研究中有着不可替代的优势.Brunner等[53]利用原位固体13C NMR方法研究了DUT-8(Ni)和SNU-9的骨架柔性对13CO2/13CH4分离的影响.发现当DUT-8(Ni)为开孔状态时,材料表现出最大的CO2吸附选择性.相比之下,当DUT-8(Ni)和SNU-9为刚性构型时,选择性最小.另外,Matoga等[54]报道了柔性JUK-8cp吸附剂在CO2/CH4分离过程中能高选择性的吸附CO2.结合固体13C NMR实验,发现在JUK-8cp孔道中存在两种不同状态的CO2:一种是吸附态,另一种是自由气态.对于芳香类混合物的分离,Li等[55]通过固体NMR实验揭示了苯乙烯/乙基苯分离在MIL-53(Al)中的主-客体相互作用.苯乙烯与MIL-53有机骨架存在的π-π相互作用导致苯乙烯相对乙基苯与MIL-53(Al)有更强的相互作用.
3 固体NMR研究MOFs中化学品的吸附的微观机制
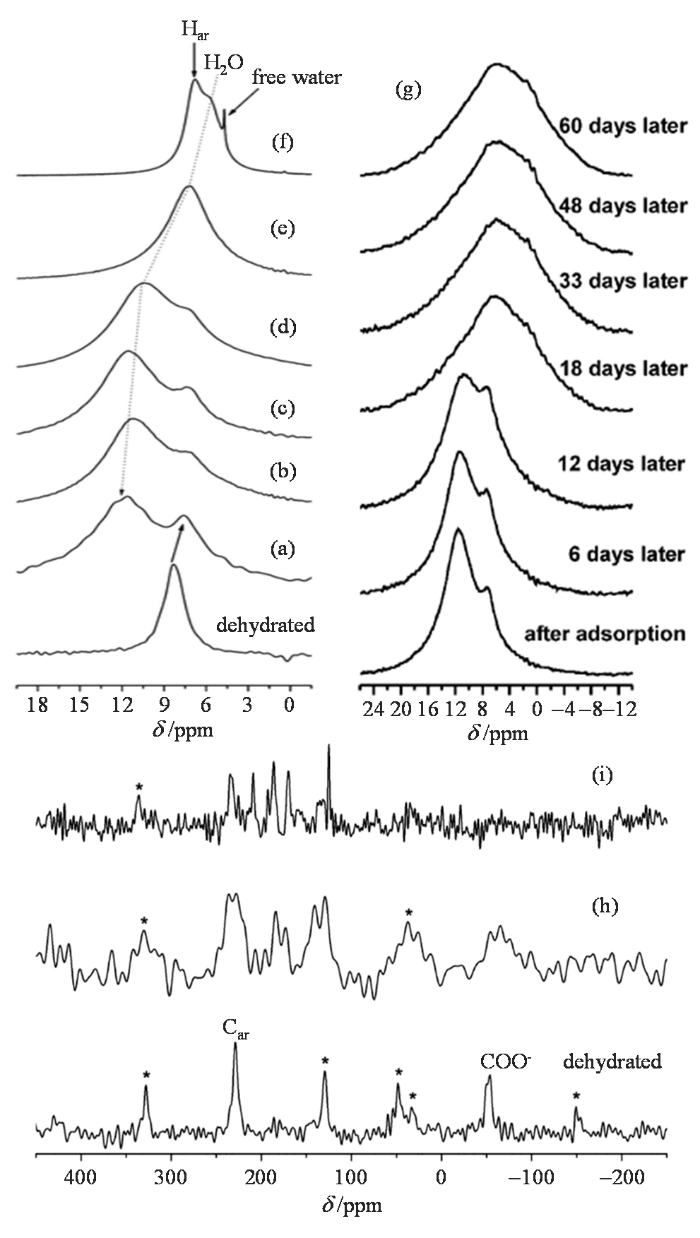
Gul-E-Noor等[57]研究了水与含不饱和金属位点的HKUST-1的主客体相互作用以及HKUST-1在水气氛下的稳定性.图5(a)~5(f)给出了活化后以及不同吸水量的HKUST-1的1H MAS NMR图谱.从图中可以明显分辨出芳环氢、吸附在开放Cu金属位点上的水,以及自由水三种质子信号.吸附在开放Cu金属位点上的水的1H NMR化学位移随着吸附量的不同而改变.在低负载量下(0.5~1.5 H2O/Cu),化学吸附以及顺磁位移效应致使吸附在Cu位点上水的化学位移出现在δH 11 [图5(a)~(d)].随着吸水量的增加,吸附在铜位点上的水的1H NMR化学位移逐渐减小,这是由于存在着水吸附/解吸的动态平衡过程.而在高的负载量下,在δH 4.7处出现了自由水[图5(f)].
图5
图5
(a)~(f) Cu3(btc)2上吸附不同水量(a) 0.5、(b) 0.75、(c) 1、(d) 1.5、(e) 2、(f) 5 H2O/Cu的1H MAS NMR谱;(g)吸附 1 H2O/Cu的Cu3(btc)2随时间变化的1H MAS NMR谱;(h)~(i)吸附不同水量 (h) 1 H2O/Cu, (i) 2 H2O/Cu的Cu3(btc)2经历一段时间后的13C单脉冲去耦NMR谱[57]
Fig. 5
(a)~(f) 1H MAS spectra of dehydrated Cu3(btc)2 and upon adsorption of (a) 0.5, (b) 0.75, (c) 1, (d) 1.5, (e) 2, and (f) 5 mole equivalents of water with respect to copper loaded on Cu3(btc)2; (g) 1H MAS NMR spectra of water (1 mole equivalents with respect to copper) loaded on Cu3(btc)2 over a period of time after adsorption; 13C direct polarization (DP) MAS spectra of (h) 1 and (i) 2 mole equivalents of water with respect to copper loaded on Cu3(btc)2 over a period of time after adsorption[57]
此外,1H和13C NMR还用来监测HKUST-1在水气氛下的稳定性.如图5(g)所示,当吸水量为1 H2O/Cu时,经过了18天,1H NMR谱图发生显著的变化,这预示着Cu3(btc)2的结构开始坍塌.13C NMR图谱进一步佐证Cu3(btc)2在长时间水气氛下的坍塌.如图5(h)所示,吸附1 H2O/Cu的Cu3(btc)2在经过21天之后,13C NMR图谱芳香碳和羧基碳的特征峰都有一定程度上的展宽.但在吸附2 H2O/Cu的HKUST-1在经历12天之后的13C NMR谱图上出现了δC 125~210处的信号[图5(i)],这表明均苯三甲酸从HKUST-1骨架中分解出来.这个工作清晰提出了Cu3(btc)2不饱和金属位点下吸附水的模型以及Cu3(btc)2在不同吸水量随时间变化的结构稳定性.
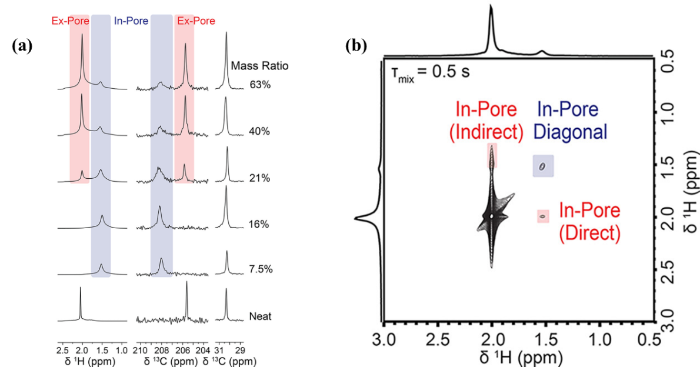
客体分子引入MOFs后往往会发生的化学环境的改变,我们根据客体分子化学位移的变化可以推断出吸附质-吸附剂上的主客体相互作用.Reimer等[62]将固体NMR技术和DFT计算相结合研究了吸附物的孔内和孔外化学位移的不同,并确定了甲醇、丙酮和环己烷在UiO-66(Zr)中的结合位点.如图6(a)所示,少量丙酮(7.5%和16%)被吸附在UiO-66(Zr)上时,丙酮的1H和13C NMR的特征峰与纯丙酮相比都发生了偏移,这些特征峰可归属为进入UiO-66(Zr)孔道内部的丙酮.当丙酮的负载量增加,孔外丙酮的特征峰开始出现.在1H和13C NMR中观察到的孔内化学位移相比孔外化学位移偏移值符号相反,暗示丙酮与Zr-OH基团之间的相互作用引起了化学位移的变化,并且丙酮优先吸附在了八面体笼中.除此之外,还通过二维1H同核相关固体NMR实验[图6(b)]确定孔内和孔外两种化学环境的丙酮存在相互交换.孔内和孔外两种不同化学环境的丙酮之间的化学交换时间常数为0.1 s量级.同时,根据环己烷和甲醇吸附在UiO-66(Zr)上的1H和13C NMR谱图,结合理论计算推测出了甲醇与丙酮一样,优先吸附在了八面体笼中.而环己烷的吸附产生的化学位移的偏移来自于与芳环相互作用产生的环电流效应引起化学位移往高场偏移,且环己烷优先吸附在UiO-66(Zr)的四面体笼中.这项工作将MOFs孔隙环境对液态客体溶剂的吸附行为的影响有效联系起来,对于理解吸附质-吸附剂相互作用机制的十分重要.
图6
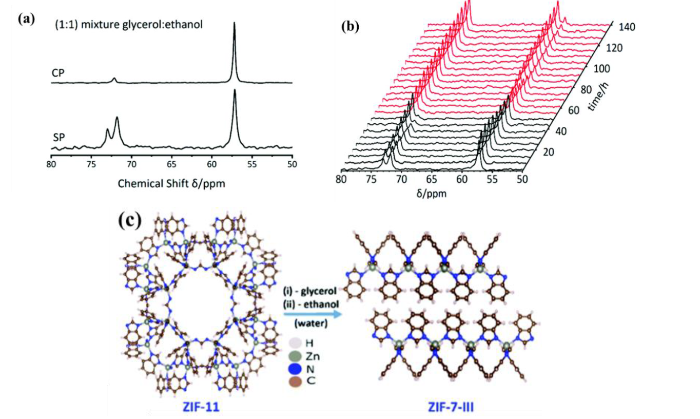
固体NMR对被探测核的局部环境很敏感,它可以通过化学位移来获取吸附物种分子结构和相互作用的信息,因此固体NMR是探究MOFs内醇类选择竞争性吸附的一个有效的途径.Brunner等[65]通过原位固体NMR研究了ZIF-8和ZIF-11两种MOFs在水溶液中对乙醇和甘油的液相竞争吸附.图7给出了乙醇和甘油共吸附在ZIF-11的单脉冲(SP)和CP 13C NMR谱,位于δC 57.2、58.3、71.8和73.2的信号分别归属为吸附乙醇、液体乙醇、吸附甘油和液体甘油的信号.吸附有1:1的乙醇和甘油混合物的ZIF-11的随时间变化的固体13C NMR图谱反映了乙醇和甘油与MOFs相互作用强度的差异[图7(b)].随着时间的增加,δC 57.2处吸附的乙醇和δC 71.8处吸附的甘油在逐渐减少,而δC 58.3处的液体乙醇和δC 73.2处的液体甘油在逐渐增加,且δC 71.8特征峰率先消失,说明吸附的乙醇和甘油逐渐脱附出来,且甘油优先从孔中完全脱附.本工作发现乙醇比甘油在MOFs孔道中的相互作用更强.通过固体13C CP/MAS NMR图谱观测到多孔ZIF-11在一段时间内发生了向无孔ZIF-7-III的相变.实验结果也说明客体分子的引入可能会引起MOFs的结构改变,进而可以推断ZIF-8和ZIF-11的稳定性.这项工作给出了观测MOFs孔道内液相客体分子吸附和脱附的成功案例.
图7
图7
(a)吸附1 mol/L 1:1甘油:乙醇溶液的ZIF-11的单脉冲和交叉极化13C NMR谱;(b) 1:1的甘油:乙醇溶液吸附在ZIF-11随时间变化的单脉冲13C NMR谱;(c) ZIF-11至ZIF-7-lll的结构转变[65]
Fig. 7
(a) 13C single pulse (SP) and cross polarization (CP) NMR spectra of ZIF-11 loaded with 1 mol/L 1:1 glycerol:ethanol solution; (b) Time-dependence of 13C SP NMR spectra of ZIF-11 loaded with 1:1 glycerol:ethanol solution; (c) Structural transformation of ZIF-7-III from ZIF-11 upon the removal of guest solvents[65]
固体NMR也常被用来识别其他化学品与MOFs吸附剂之间的相互作用.Xu等[66]采用多核固体NMR方法研究了MOF-56-H2与罗丹明B(RhB)之间的相互作用机制主要是MOF-56-H2骨架与多环烃之间的π-π相互作用.Wittmann等[67]利用固体NMR探究了水、二乙胺、2-氨基吡啶和3-氨基吡啶与Cr-MIL-101中不饱和金属位点的吸附行为,发现配位结合强度顺序为:水<二乙胺≈2-氨基吡啶<3-氨基吡啶.Pourpoint等[68]利用两维异核相关实验证实了DMF分子会与UiO-66(Zr)中的Zr-OH基团形成氢键.Jeong等[69]通过1H NMR观察到了HKUST-1不饱和金属位点上多种溶剂分子存在多重配位交换的现象,这一研究发现将有助于去除不饱和金属位点上的强配位溶剂(如DMF、N,N-二乙基甲酰胺和二甲基亚砜).这些研究为加深理解液相分子与MOFs的相互作用微观机制提供了指引.
4 总结
固体NMR已被广泛应用于探究MOFs体系吸附、分离过程中的主客体相互作用机制.在气体吸附方面,变温静态固体2H、13C、17O NMR谱可以给出吸附气体在MOFs孔道内的吸附方式、动力学行为和相互作用强弱等重要的微观信息.同时,固体NMR还可以得到气体分子与MOFs孔道内优先吸附位点和扩散速率等.在低碳烷烃/烯烃分离方面,固体NMR可以用来测定混合气体在MOFs中的分离选择性,并能从微观上观测混合气体在MOFs孔道中的竞争优先吸附,从而得到MOFs气体分离的微观机制.另外,固体NMR还可用来揭示常见化学品在MOFs孔道内的相互作用模式.这些研究发现将有助于理解MOFs在吸附分离过程中的构效关系.除了吸附和分离,固体NMR在研究MOFs在传感、催化、药物输运等方面的主客体相互作用机制也取得一些重要的进展.灵敏度增强的谱仪技术(高场谱仪、超高速魔角旋转和动态核极化(DNP))和脉冲方法(高效脉冲重耦和去耦)的运用,以及高压转子和微成像技术的不断发展和进步,将会进一步推进和拓展固体NMR在研究MOFs主客体化学上的应用.
致谢
感谢中国科学院磁共振技术联盟功能开发项目(2020gz1007)的资助.
利益冲突
无
参考文献
The chemistry and applications of metal-organic frameworks
[J].
DOI:10.1126/science.1230444
URL
[本文引用: 1]

\n Metal-organic frameworks (MOFs) are made by linking inorganic and organic units by strong bonds (reticular synthesis). The flexibility with which the constituents’ geometry, size, and functionality can be varied has led to more than 20,000 different MOFs being reported and studied within the past decade. The organic units are ditopic or polytopic organic carboxylates (and other similar negatively charged molecules), which, when linked to metal-containing units, yield architecturally robust crystalline MOF structures with a typical porosity of greater than 50% of the MOF crystal volume. The surface area values of such MOFs typically range from 1000 to 10,000 m\n 2\n /g, thus exceeding those of traditional porous materials such as zeolites and carbons. To date, MOFs with permanent porosity are more extensive in their variety and multiplicity than any other class of porous materials. These aspects have made MOFs ideal candidates for storage of fuels (hydrogen and methane), capture of carbon dioxide, and catalysis applications, to mention a few.\n
Introduction to metal-organic frameworks
[J].DOI:10.1021/cr300014x URL [本文引用: 1]
Solid-state NMR studies of internuclear correlations for characterizing catalytic materials
[J].DOI:10.1039/D0CS01130D URL [本文引用: 1]
Understanding surface and interfacial chemistry in functional nanomaterials via solid-state NMR
[J].DOI:10.1002/adma.v29.14 URL [本文引用: 1]
Progresses of hyperpolarized 129Xe NMR application in porous materials and catalysis
[J].
Analysis of local structure, acidic property and activity of solid acids by solid-state nuclear magnetic resonance spectroscopy
[J].
基于固体核磁共振技术的固体酸结构、酸性及活性分析
[J].
Progress in the studies on Sn-zeolites by solid-state nuclear magnetic resonance
[J].
固体核磁共振技术在锡硅分子筛表征中的应用
[J].
Amine dynamics in diamine-appended Mg2 (dobpdc) metal-organic frameworks
[J].DOI:10.1021/acs.jpclett.9b02883 URL [本文引用: 2]
Solid-state NMR studies of sulfonated SBA-15 and the synergistic catalysis of fructose into 5-hydroxymethylfurfural with dimethyl sulfoxide
[J].
Application of ammonia probe-assisted solid-state NMR technique in zeolites and catalysis
[J].
Structure and acidity changes in ultra-stable Y zeolites during hydrothermal aging: A solid-state NMR spectroscopy study
[J].
NMR研究超稳Y分子筛水热老化过程中结构与酸性的变化
[J].
The effects of ammonium hexafluorosilicate post-treatment on the acidity of H-ZSM-5 zeolite studied by solid-state NMR spectroscopy
[J].
六氟硅酸铵后处理对H-ZSM-5分子筛酸性影响的固体NMR研究
[J].
Solid-state NMR spectroscopy: A powerful technique to directly study small gas molecules adsorbed in metal-organic frameworks
[J].DOI:10.1002/chem.v25.8 URL [本文引用: 1]
Solid-state NMR spectroscopy: an advancing tool to analyse the structure and properties of metal-organic frameworks
[J].
DOI:10.1039/d0sc00735h
PMID:34122887
[本文引用: 1]

Metal-organic frameworks (MOFs) gain increasing interest due to their outstanding properties like extremely high porosity, structural variability, and various possibilities for functionalization. Their overall structure is usually determined by diffraction techniques. However, diffraction is often not sensitive for subtle local structural changes and ordering effects as well as dynamics and flexibility effects. Solid-state nuclear magnetic resonance (ssNMR) spectroscopy is sensitive for short range interactions and thus complementary to diffraction techniques. Novel methodical advances make ssNMR experiments increasingly suitable to tackle the above mentioned problems and challenges. NMR spectroscopy also allows study of host-guest interactions between the MOF lattice and adsorbed guest species. Understanding the underlying mechanisms and interactions is particularly important with respect to applications such as gas and liquid separation processes, gas storage, and others. Special NMR experiments allow investigation of properties and functions of MOFs under controlled and application-relevant conditions. The present minireview explains the potential of various solid-state and NMR techniques and illustrates their application to MOFs by highlighting selected examples from recent literature.This journal is © The Royal Society of Chemistry.
Solid-state NMR of small molecule adsorption in metal-organic frameworks (MOFs)
[M].
Probing molecular motions in metal-organic frameworks with solid-state NMR
[J].DOI:10.1016/j.ccr.2020.213563 URL [本文引用: 1]
Variable temperature and pressure operando MAS NMR for catalysis science and related materials
[J].DOI:10.1021/acs.accounts.9b00557 URL [本文引用: 1]
Solid-state NMR investigations of carbon dioxide gas in metal-organic frameworks: Insights into molecular motion and adsorptive behavior
[J].
DOI:10.1021/acs.chemrev.7b00695
PMID:30288971
[本文引用: 1]

Solid-state nuclear magnetic resonance (SSNMR) methods have been routinely used for the characterization of both the structure and the dynamics of metal organic frameworks (MOFs), a collection of porous media investigated for potential applications in carbon capture technologies, selective separation of small molecules, and catalysis. (1) The use and development of SSNMR techniques that enable the nondestructive characterization of the adsorbed behavior have become essential steps in bettering our understanding of MOFs and are often complementary to traditional methods of structural characterization. This Review aims to give a brief introduction to the relevant concepts of SSNMR and the methods employed when investigating the phenomenon of adsorbed carbon dioxide gas in MOFs. We summarize the published SSNMR literature on CO in MOFs, as well as highlight the best experimental practices when working with these complex systems.
Characterization of metal-organic frameworks: Unlocking the potential of solid-state NMR
[J].DOI:10.1021/acs.accounts.7b00357 URL [本文引用: 1]
The role of NMR in metal organic frameworks: deep insights into dynamics, structure and mapping of functional groups
[J].
Application of solid-state NMR techniques for structural characterization of metal-organic frameworks
[J].DOI:10.1016/j.ssnmr.2022.101772 URL [本文引用: 1]
Two open metal sites on the same metal: Dynamics of CO2 in MOF UTSA-74
[J].
Recent advances of solid-state NMR spectroscopy for microporous materials
[J].DOI:10.1002/adma.v32.44 URL [本文引用: 1]
O-17 NMR spectroscopy of crystalline microporous materials
[J].DOI:10.1039/D1SC00552A URL [本文引用: 1]
Combined solid-state NMR, FT-IR and computational studies on layered and porous materials
[J].
DOI:10.1039/c7cs00358g
PMID:30014075
[本文引用: 1]

Understanding the structure-property relationship of solids is of utmost relevance for efficient chemical processes and technological applications in industries. This contribution reviews the concept of coupling three well-known characterization techniques (solid-state NMR, FT-IR and computational methods) for the study of solid state materials which possess 2D and 3D architectures and discusses the way it will benefit the scientific communities. It highlights the most fundamental and applied aspects of the proactive combined approach strategies to gather information at a molecular level. The integrated approach involving multiple spectroscopic and computational methods allows achieving an in-depth understanding of the surface, interfacial and confined space processes that are beneficial for the establishment of structure-property relationships. The role of ssNMR/FT-IR spectroscopic properties of probe molecules in monitoring the strength and distribution of catalytic active sites and their accessibility at the porous/layered surface is discussed. Both experimental and theoretical aspects will be considered by reporting relevant examples. This review also identifies and discusses the progress, challenges and future prospects in the field of synthesis and applications of layered and porous solids.
Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II)
[J].DOI:10.1021/jacs.5b10754 URL [本文引用: 1]
Molecular rotors in porous organic frameworks
[J].DOI:10.1002/anie.201309362 URL [本文引用: 1]
Grasping hydrogen adsorption and dynamics in metal-organic frameworks using 2H solid-state NMR
[J].DOI:10.1039/C6CC03205B URL [本文引用: 1]
A multifaceted study of methane adsorption in metal-organic frameworks by using three complementary techniques
[J].DOI:10.1002/chem.v24.31 URL [本文引用: 1]
Analyzing gas adsorption in an amide-functionalized metal organic framework: are the carbonyl or amine groups responsible?
[J].DOI:10.1021/acs.chemmater.8b00681 URL [本文引用: 3]
UiO-66 (Zr) MOF as a promising material for butane isomers separation: Evidence based on the analysis of the adsorbed alkanes mobility by 2H NMR and molecular dynamics simulation
[J].DOI:10.1021/acs.jpcc.1c02849 URL [本文引用: 1]
Solid-state NMR studies of host-guest interaction between UiO-67 and light alkane at room temperature
[J].DOI:10.1021/acs.jpcc.7b04611 URL [本文引用: 1]
Primary adsorption sites of light alkanes in multivariate UiO-66 at room temperature as revealed by solid-state NMR
[J].DOI:10.1021/acs.jpcc.0c00184 URL [本文引用: 1]
Monitoring the diffusivity of light hydrocarbons in a mixture by magic angle spinning pulsed field gradient NMR: methane/ethane/ethene in ZIF-8
[J].DOI:10.1021/acs.jpcc.7b09335 URL [本文引用: 1]
Ethene/ethane mixture diffusion in the MOF sieve ZIF-8 studied by MAS PFG NMR diffusometry
[J].DOI:10.1016/j.micromeso.2011.06.009 URL [本文引用: 1]
Anomalous relationship between molecular size and diffusivity of ethane and ethylene inside crystals of zeolitic imidazolate framework-11
[J].DOI:10.1021/acs.jpcc.9b03933 URL [本文引用: 1]
NMR study of the host structure and guest dynamics investigated with alkane/alkene mixtures in metal organic frameworks ZIF-8
[J].DOI:10.1021/acs.jpcc.8b11673 URL [本文引用: 1]
Self-diffusion studies in CuBTC by PFG NMR and MD simulations
[J].DOI:10.1021/jp102212w URL [本文引用: 1]
CO2 dynamics in a metal-organic framework with open metal sites
[J].DOI:10.1021/ja306822p URL [本文引用: 1]
Wobbling and hopping: studying dynamics of CO2 adsorbed in metal-organic frameworks via 17O solid-state NMR
[J].DOI:10.1021/jz501729d URL [本文引用: 4]
Sizable dynamics in small pores: CO2 location and motion in the α-Mg formate metal-organic framework
[J].DOI:10.1039/C7CP00199A URL [本文引用: 2]
Deducing CO2 motion, adsorption locations and binding strengths in a flexible metal-organic framework without open metal sites
[J].DOI:10.1039/C5CP04984A URL [本文引用: 2]
Exploring host-guest interactions in the α-Zn3 (HCOO)6 metal-organic framework
[J].DOI:10.1021/acsomega.8b03623 URL [本文引用: 1]
Understanding the fascinating origins of CO2 adsorption and dynamics in MOFs
[J].DOI:10.1021/acs.chemmater.6b02239 URL [本文引用: 1]
Adsorption of small molecules on Cu3(btc)2 and Cu3-xZnx(btc)2 metal-organic frameworks (MOF) as studied by solid-state NMR
[J].DOI:10.1021/jp400869f URL [本文引用: 2]
CO2 behavior in a highly selective ultramicroporous framework: insights from single-crystal X-ray diffraction and solid-state nuclear magnetic resonance spectroscopy
[J].DOI:10.1021/acs.jpcc.9b03221 URL [本文引用: 1]
A diaminopropane-appended metal-organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism
[J].DOI:10.1021/jacs.7b07612 URL [本文引用: 1]
Elucidating CO2 chemisorption in diamine-appended metal-organic frameworks
[J].DOI:10.1021/jacs.8b10203 URL [本文引用: 1]
Water enables efficient CO2 capture from natural gas flue emissions in an oxidation-resistant diamine-appended metal-organic framework
[J].DOI:10.1021/jacs.9b05567 URL [本文引用: 1]
Overcoming metastable CO2 Adsorption in a bulky diamine-appended metal-organic framework
[J].DOI:10.1021/jacs.1c06434 URL [本文引用: 1]
Host-guest interaction in ethylene and ethane separation on zeolitic imidazolate frameworks as revealed by solid-state NMR spectroscopy
[J].DOI:10.1002/chem.v27.44 URL [本文引用: 3]
Preferential adsorption sites for propane/propylene separation on ZIF-8 as revealed by solid-state NMR spectroscopy
[J].
DOI:10.1039/d1cp05931a
PMID:35258049
[本文引用: 3]

Solid-state NMR spectroscopy in conjunction with theoretical calculation was employed to investigate the adsorbent-adsorbate host-guest interactions during propane/propylene separation on ZIF-8. H NMR chemical shifts of free gaseous and adsorbed propane/propylene are unambiguously assigned with the assistance of two-dimensional (2D) H-H correlation spectroscopy (COSY) MAS NMR spectra. Meanwhile, the adsorption selectivity for propane/propylene mixtures on ZIF-8 at a pressure in range of 1.9-9.6 bar is quantitatively determined using H MAS NMR experiments, which agreed well with the ideal adsorbed solution theory (IAST) predictions. The preferential adsorption of propane compared with propylene on ZIF-8 is directly visualized from the 2D H-H spin diffusion homo-nuclear correlation (HOMCOR) MAS NMR spectroscopy. Moreover, the preferential adsorption sites for propane and propylene are deduced from the H-H spin diffusion buildup curves, which is further confirmed by DFT theoretical calculations. This work provides insights to understand the structure-property relationship during the propane/propylene separation on ZIF-8 as adsorbent.
In situ 13C NMR spectroscopy study of CO2/CH4 mixture adsorption by metal-organic frameworks: does flexibility influence selectivity?
[J].DOI:10.1021/acs.langmuir.8b03554 URL [本文引用: 1]
Combining in situ techniques (XRD, IR, and 13C NMR) and gas adsorption measurements reveals CO2-induced structural transitions and high CO2/CH4 selectivity for a flexible metal-organic framework JUK-8
[J].DOI:10.1021/acsami.1c07268 URL [本文引用: 1]
Host-guest interaction of styrene and ethylbenzene in MIL-53 studied by solid-state NMR
[J].
DOI:S0926-2040(17)30150-9
PMID:29316473
[本文引用: 1]

Solid-state NMR was utilized to explore the host-guest interaction between adsorbate and adsorbent at atomic level to understand the separation mechanism of styrene (St) and ethylbenzene (EB) in MIL-53(Al). C-Al double-resonance NMR experiments revealed that the host-guest interaction between St and MIL-53 was much stronger than that of EB adsorption. In addition, C DIPSHIFT experiments suggested that the adsorbed St was less mobile than EB confined inside the MIL-53 pore. Furthermore, the host-guest interaction model between St, EB and MIL-53 was established on the basis of the spatial proximities information extracted from 2D H-H homo-nuclear correlation NMR experiments. According to the experimental observation from solid-state NMR, it was found that the presence of π-π interaction between St and MIL-53 resulted in the stronger host-guest interaction and less mobility of St. This work provides direct experimental evidence for understanding the separation mechanism of St and EB using MIL-53 as an adsorbent.Copyright © 2017 Elsevier Inc. All rights reserved.
25Mg solid-state NMR: a sensitive probe of adsorbing guest molecules on a metal center in metal-organic framework CPO-27-Mg
[J].DOI:10.1021/jz301954t URL [本文引用: 1]
Effects of varying water adsorption on a Cu3(BTC)2 metal-organic framework (MOF) as studied by 1H and 13C solid-state NMR spectroscopy
[J].DOI:10.1039/c0cp02848g URL [本文引用: 4]
The surprising stability of Cu3(btc)2 metal-organic framework under steam flow at high temperature
[J].DOI:10.1021/acs.cgd.8b00931 URL [本文引用: 1]
Time dependent water uptake in Cu3(btc)2 MOF: Identification of different water adsorption states by 1H MAS NMR
[J].DOI:10.1016/j.micromeso.2013.06.033 URL [本文引用: 2]
Hydrolytic stability in hemilabile metal-organic frameworks
[J].
DOI:10.1038/s41557-018-0104-x
PMID:30104722
[本文引用: 1]

Highly porous metal-organic frameworks (MOFs), which have undergone exciting developments over the past few decades, show promise for a wide range of applications. However, many studies indicate that they suffer from significant stability issues, especially with respect to their interactions with water, which severely limits their practical potential. Here we demonstrate how the presence of 'sacrificial' bonds in the coordination environment of its metal centres (referred to as hemilability) endows a dehydrated copper-based MOF with good hydrolytic stability. On exposure to water, in contrast to the indiscriminate breaking of coordination bonds that typically results in structure degradation, it is non-structural weak interactions between the MOF's copper paddlewheel clusters that are broken and the framework recovers its as-synthesized, hydrated structure. This MOF retained its structural integrity even after contact with water for one year, whereas HKUST-1, a compositionally similar material that lacks these sacrificial bonds, loses its crystallinity in less than a day under the same conditions.
Formation of mixed metal Cu3-xZnx(btc)2 frameworks with different zinc contents: Incorporation of Zn2+ into the metal-organic framework structure as studied by solid-state NMR
[J].DOI:10.1021/jp3054857 URL [本文引用: 1]
NMR spectroscopy reveals adsorbate binding sites in the metal-organic framework UiO-66 (Zr)
[J].DOI:10.1021/acs.jpcc.7b12628 URL [本文引用: 3]
Breathing effect via solvent inclusions on the linker rotational dynamics of functionalized MIL-53
[J].DOI:10.1002/chem.v27.59 URL [本文引用: 1]
13C chemical shift tensors in MOF α-Mg3(HCOO)6: Which component is more sensitive to host-guest interaction?
[J].
Solid-state NMR insights into alcohol adsorption by metal-organic frameworks: adsorption state, selectivity, and adsorption-induced phase transitions
[J].
DOI:10.1039/D2CC00638C
URL
[本文引用: 3]

Alcohol adsorption by metal–organic frameworks (ZIF-8 and ZIF-11) in aqueous solutions is investigated including alcohol mixtures.
Isolated π-interaction sites in mesoporous MOF backbone for repetitive and reversible dynamics in water
[J].DOI:10.1021/acsami.8b19211 URL [本文引用: 1]
Probing interactions of N-donor molecules with open metal sites within paramagnetic Cr-MIL-101: a solid-state NMR spectroscopic and density functional theory study
[J].
DOI:10.1021/jacs.7b10148
PMID:29316398
[本文引用: 1]

Understanding host-guest interactions is one of the key requirements for adjusting properties in metal-organic frameworks (MOFs). In particular, systems with coordinatively unsaturated Lewis acidic metal sites feature highly selective adsorption processes. This is attributed to strong interactions with Lewis basic guest molecules. Here we show that a combination of C MAS NMR spectroscopy with state-of-the-art density functional theory (DFT) calculations allows one to unravel the interactions of water, 2-aminopyridine, 3-aminopyridine, and diethylamine with the open metal sites in Cr-MIL-101. The C MAS NMR spectra, obtained with ultrafast magic-angle spinning, are well resolved, with resonances distributed over 1000 ppm. They present a clear signature for each guest at the open metal sites. Based on competition experiments this leads to the following binding preference: water < diethylamine ≈ 2-aminopyridine < 3-aminopyridine. Assignments were done by exploiting distance sum relations derived from spin-lattice relaxation data and C{H} REDOR spectral editing. The experimental data were used to validate NMR shifts computed for the Cr-MIL-101 derivatives, which contain CrO clusters with magnetically coupled metal centers. While both approaches provide an unequivocal assignment and the arrangement of the guests at the open metal sites, the NMR data offer additional information about the guest and framework dynamics. We expect that our strategy has the potential for probing the binding situation of adsorbate mixtures at the open metal sites of MOFs in general and thus accesses the microscopic interaction mechanisms for this important material class, which is essential for deriving structure-property relationships.
Probing adsorption of water and DMF in UiO-66 (Zr) using solid-state NMR
[J].DOI:10.1016/j.ssnmr.2022.101797 URL [本文引用: 1]
Multiple coordination exchanges for room-temperature activation of open-metal sites in metal-organic frameworks
[J].DOI:10.1021/acsami.7b07299 URL [本文引用: 1]