引言
具有微纳结构的多孔材料在催化、吸附、绝缘、储能、分离与提纯等诸多领域中有着广泛应用[1].建立此类材料的结构与性能关系,进而进行材料设计的关键在于对其微纳结构,特别是微纳结构尺寸的定量表征.然而,由于多孔材料通常具有复杂的微观结构,确定空隙的大小、形貌、分布以及相互之间的连通性往往充满挑战,特别是在纳米尺度上.
2010年,Anderson等人[9]在使用原位氢气负载的高压核磁共振实验中发现,多孔碳质材料的不饱和键会在磁场中产生环流并进而产生诱导磁场,受诱导磁场影响的氢气分子的化学位移因此会发生偏移,并与材料的氢气负载能力呈现相关.相较于氙气等探针分子,氢气具有核磁共振灵敏度高、成本低廉,对微孔结构的穿透性强等优点,但氢气能否像氙气一样在微纳结构表征上得到实际应用,还有赖于对其谱学特征与结构关系的系统研究.
本文选择了一系列具有不同尺寸、结构规整简单的二氧化硅小球作为实验样品,观察负载氢气之后,小球堆积空隙中氢气的核磁共振谱学特征,通过多种核磁共振技术并结合扫描电镜等分析技术探讨了氢气谱学特征产生的机制及其与微纳结构尺寸的关系.
1 实验部分
1.1 试剂与样品
材料准备:实验所用的100 nm、5 μm、10 μm、100 μm粒径的二氧化硅微球(SiO2,上海辉质生物科技有限公司)经过马弗炉600 ℃加热400 min以除去所含水分.实验所用纳米孔径的多孔二氧化硅材料(SiO2,99.5%,325 mesh,Adamas)也用相同方式烘干处理,经布鲁诺-爱米特-泰勒(Brunauer-Emmett-Teller,BET)比表面积测定法测得,该样品的孔径为3 nm,比表面积为132 m2/g.实验用氢气由纯水氢气发生器(TH-300,北京中惠普)制备并收集于氢气钢瓶.
核磁共振样品:若无特殊说明,使用的核磁共振样品均是在含Teflon垫的5 mm的螺口核磁共振样品管中,添加特定尺寸的二氧化硅材料至检测区间的上限,并通入0.2 MPa的氢气得到.在相同核磁共振样品管中通入0.2 MPa氢气,作为对照用样品1;在核磁共振样品管中添加纳米孔径的多孔二氧化硅至检测区间的上限,并注入0.2 MPa氢气,作为样品2;添加纳米孔径的多孔二氧化硅至检测区间中线并注入0.2 MPa氢气,作为样品3.
1.2 实验仪器与参数
核磁共振实验与参数设置:核磁共振实验均在Bruker AVANCE III 500 MHz NMR仪器上进行,使用探头为Bruker双共振宽带高分辨探头.无特殊说明,实验温度均为298 K.核磁共振实验前均使用水信号作为外标,将水峰定为4.7 ppm,并给予后续谱图以相同的频偏以定标.核磁共振氢谱均由单脉冲激发,循环等待时间为4 s.文中的氢谱均为与背景信号的差谱,同一实验中的氢谱均使用相同的谱图后处理参数.二维交换实验所用序列为NOESY序列,其中混合时间τ设为0.1 ms.使用受激回波(Stimulated Echo,STE)序列测量氢气的自扩散速率,其中梯度脉宽δ设置为800 μs,扩散时间Δ为1.5 ms,梯度场最大强度为 53.75×10-4 T/cm,选取梯度强度的2%~10%,取16个梯度间隔采样.
基础材料表征:使用HITACHI S-4800冷场电子显微镜观测微纳尺寸二氧化硅形貌.
2 结果与讨论
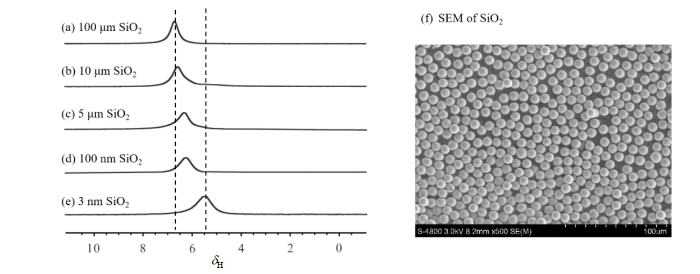
图1(a)~(d)是不同粒径(100 μm、10 μm、5 μm和100 nm)二氧化硅微球样品中氢气的核磁共振谱图.四个样品的谱图中都仅有1个共振信号.我们将该信号归属为存在于二氧化硅微球堆积形成的空隙内的氢气分子[10],后文将对此做进一步实验论证.分析这些谱图可以发现氢气信号的化学位移随着二氧化硅微球粒径变小而逐渐变小.在100 μm、10 μm、5 μm、100 nm的二氧化硅材料中,氢气信号的化学位移分别为6.5 ppm、6.4 ppm、6.1 ppm、6.0 ppm.由于这些实心二氧化硅微球表面结构相同,差别只在于尺寸,因此图1(a)~(e)中信号化学位移的变化趋势应该与氢气存在空间的尺寸大小,即二氧化硅微球紧密堆积产生的空隙大小[图1(f)]相关.为验证这一点,我们选取了平均孔径为3 nm的二氧化硅多孔材料样品并用同样方法进行测试,观察到该样品中氢气分子的化学位移为5.5 ppm [图1(e)].由于在该样品中氢气分子主要存在于样品纳米孔道之中,因此这一结果支持之前的分析,即氢气分子所处的空间尺寸越小,氢气分子的化学位移越小.随着二氧化硅微球之间空隙逐渐减小,氢气信号的半高宽逐渐增大,该现象可能与氢气分子的运动变化相关.与自由氢气不同,二氧化硅空隙中部分氢气分子的运动受到了材料限制,不同位置氢气分子间的交换作用也因此受到阻碍.随着材料尺寸减小,交换速率愈加缓慢,氢气信号因此增宽.
图1
图1
不同粒径二氧化硅微球样品中氢气的1H NMR谱图. (a)~(d)分别对应于粒径为100 μm、10 μm、5 μm、100 nm的二氧化硅微球中的氢气谱图;(e)平均孔径为3 nm的多孔二氧化硅样品中的氢气谱图;(f)粒径为100 μm的二氧化硅微球的扫描电镜照片
Fig. 1
1H NMR spectra of hydrogen gas in the stacking interstices of silica microsphere samples with different particle sizes: (a)~(d) correspond to the spectra of hydrogen gas in silica microspheres with particle sizes of 100 μm, 10 μm, 5 μm, and 100 nm, respectively; (e) shows the spectrum of hydrogen gas in a porous silica sample with an average pore size of 3 nm; (f) scanning electron microscope (SEM) image of silica microspheres with a diameter of 100 μm
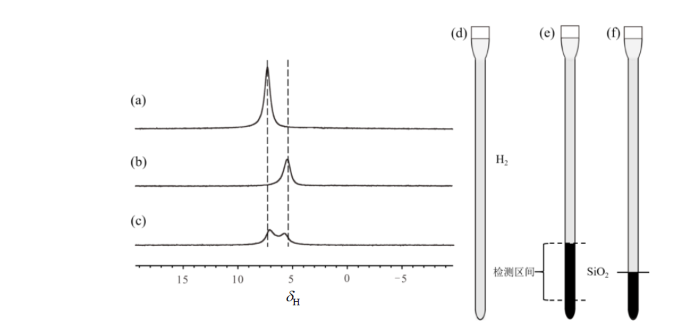
为进一步验证图1中氢气分子信号的来源,我们开展了系列对照实验.图2(a)是样品1,即未加入二氧化硅颗粒的样品中氢气的谱图,图中只有一个共振信号,化学位移为7.3 ppm,半高宽约为320 Hz.根据Fujiwara等人[11]对氢气信号的分析结果,我们将其归属为“自由”氢气信号.图2(b) [同图1(e)]是样品2的谱图,图中同样只有一个质子共振信号,化学位移为5.5 ppm,半高宽约为370 Hz,对应于多孔二氧化硅纳米孔道中的氢气信号.由于氢气分子仅占据由固体颗粒密堆积产生的空隙,图2(b)中的氢气信号强度显著弱于图2(a)中纯氢气信号.图2(c)是样品3的谱图,图中出现两个部分重叠的质子信号,化学位移分别为7.1 ppm和5.7 ppm.分峰拟合发现5.7 ppm处信号较7.3 ppm处信号宽.显然,这两个信号可归属为无二氧化硅颗粒部位的氢气信号(7.1 ppm)与二氧化硅颗粒空隙中的氢气信号(5.7 ppm).
图2
图2
三个对照样品的核磁共振氢谱及样品示意图. (a)样品1的谱图;(b)样品2的谱图;(c)样品3的谱图;(d)~(f)分别为样品1、2和3的示意图,其中黑色阴影代表加入的二氧化硅
Fig. 2
1H NMR spectra and schematic diagrams of three control samples. (a) Spectrum of Sample 1; (b) spectrum of Sample 2; (c) spectrum of Sample 3; (d)~(f) schematic diagrams of Sample 1, 2, and 3, respectively, where the black shading represents added silica dioxide
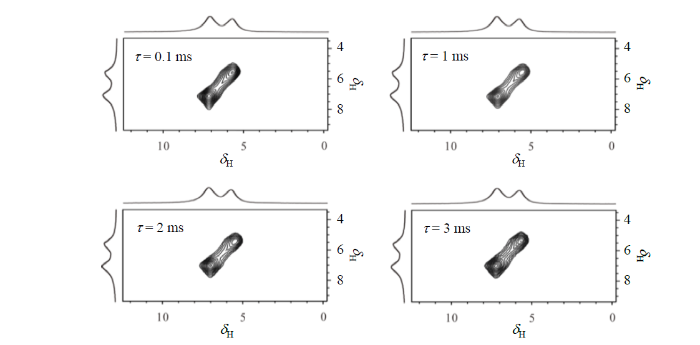
图3
图3
不同混合时间下,样品3中氢气分子的1H二维交换谱
Fig. 3
2D 1H exchange spectrum of hydrogen gas molecules of Sample 3 at different mixing time
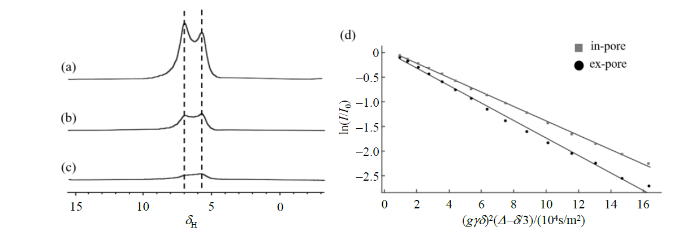
图4
图4
样品3的自扩散系数测定实验结果. (a)~(c)分别对应在2%、7%、10%梯度强度下的氢气扩散谱;(d)空隙内外两种氢分子信号强度ln(I/I0)随(gγδ)2(Δ-δ/3)的变化. 图中“in-pore”代表空隙内氢气分子信号强度,“ex-pore”代表空隙外氢气分子信号强度
Fig. 4
The experimental results of the self-diffusion coefficient determination for Sample 3: (a)~(c) correspond to the diffusion spectra of hydrogen gas under diffusion gradient strengths of 2%, 7%, and 10%, respectively; (d) the relationship between the ln(I/I0) signal intensity of two types of hydrogen molecules (inside and outside the nano pores) and (gγδ)2(Δ-δ/3). The term 'in-pore' in the figure refers to the signal intensity of hydrogen molecules inside the pores, while 'ex-pore' refers to the signal intensity of hydrogen molecules outside the pores
其中,I代表不同梯度场强度下扩散谱中氢气信号强度,
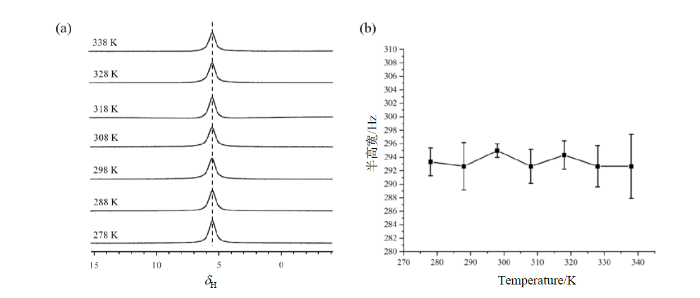
根据文献报道[13],氙气的化学位移和氙气所处的空间尺寸存在相关关系,这与图1中氢气分子的谱学性质相似.氙气因为其结构特性,很容易在材料表面吸附,吸附状态氙气信号的化学位移不同于气体状态的信号,由于氙气可以在吸附状态和气体状态之间快速交换,所以实验观察到的氙气信号是两种状态的平均[14].因为氙气所处的空间尺寸越小,吸附状态占比越大,所观察到信号就越靠近吸附状态的信号,所以氙气信号的化学位移和所处空间的尺寸存在相关关系.为了证明这种机制是否也适用于解释图1中观察到的氢气的化学位移随二氧化硅小球尺寸变化而变化的实验现象,我们开展了变温实验,如果氢气信号随着温度升高而变化,则说明氢气在二氧化硅表面可能存在吸附,因为气体的吸附能力会随温度升高而单调降低;反之,如果氢气信号随着温度升高不发生变化,则说明吸附不是导致氢气化学位移随所处空间尺寸变化而变化的原因.
图5
图5
纳米孔径二氧化硅材料中氢气分子的变温核磁共振实验结果. (a)氢气在不同温度下的1H谱;(b)氢气信号半高宽随温度的变化
Fig. 5
The variable temperature 1H NMR experimental results of hydrogen gas in nanoporous silica sample. (a) 1H spectrum of hydrogen gas at different temperatures; (b) the correlation between the half-width of the hydrogen signal and temperature
二氧化硅是一种抗磁性物质,这意味着在静磁场中,二氧化硅会发生磁化并产生与静磁场方向相反的感应磁场,其大小与静磁场强度成正比,两者间的比例常数即为磁化率[15],抗磁性物质的磁化率不随温度变化,距离颗粒越远,感应磁场越小[16].根据文献报道[17],二氧化硅的磁化率为-0.9108×10-6,会诱导产生约1 ppm的化学位移偏移.基于此,我们认为本文中观察到的实验现象是由两个因素共同作用所导致的,即:1)抗磁性的二氧化硅颗粒在静磁场中产生的感应磁场导致的磁场不均匀分布;2)感受到不同感应磁场强度的氢气分子之间的快速交换.具体而言,靠近二氧化硅颗粒的氢气分子感受到较强的与静磁场方向相反的感应磁场,信号出现在相对高场位置;距离二氧化硅颗粒较远的氢气分子没有感受到,或者感受到相对较小的感应磁场,信号出现在相对低场位置;由于氢气分子的快速运动,这两类氢气分子处于快速的交换之中,所以实验中观察到的始终是两种状态的氢气分子信号加权平均的结果,信号的化学位移取决于两种状态氢气分子的相对含量,二氧化硅颗粒尺寸越小,紧密堆积产生的空隙越小,靠近二氧化硅颗粒的氢气分子相对含量越高,信号的化学位移也就越小.由于抗磁性物质的磁化率不随温度变化,所以相应的氢气信号化学位移也应该不随温度变化.抗磁性是微纳结构材料的普遍特性,氢气分子的化学位移随所处空间的尺寸变化而变化的现象有望被应用于材料中微纳结构尺寸的测量,后续我们将选择更多的样品体系开展相关研究.
3 结论
本文研究了不同尺寸的二氧化硅小球堆积形成的空隙中氢气分子的1H NMR信号特征.观察到氢气分子的化学位移随着二氧化硅小球堆积空隙减小而往高场方向移动的现象.结合变温氢谱、二维交换谱及扫描电镜等实验结果,对该现象产生的机制进行了分析,提出了抗磁性的二氧化硅导致的磁场不均匀性和处于不同位置的氢气分子的快速交换两个因素的共同作用是该现象产生的原因的观点,由于氢气分子化学位移数值和二氧化硅小球堆积产生的空隙尺寸间存在明显的相关性,故本文的结果说明氢气作为探针分子在微纳尺寸测量中具备潜在的应用价值.
利益冲突
无
参考文献
Pore classification in the characterization of porous materials: A perspective
[J].
Ultramicropores in microporous carbon fibres evidenced by helium adsorption at 4.2 K
[J].
Scattering by an inhomogeneous solid. II. The correlation function and its application
[J].
Chemistry in noninteger dimensions between two and three. I. Fractal theory of heterogeneous surfaces
[J].
Alkoxide silica gel: Porous structure by thermoporometry
[J].
Study on the automatic accumulation-thawing device of hyperpolarized 129Xe
[J].
超极化129Xe自动收集-升华装置研究
[J].
DOI:10.11938/cjmr20222998
[本文引用: 1]

因其较高的核自旋极化度所提供的探测灵敏度,超极化<sup>129</sup>Xe气体已被成功应用于动物和人体磁共振成像(MRI).但是,在超极化<sup>129</sup>Xe的收集-升华过程中,多种因素会导致<sup>129</sup>Xe核自旋弛豫,进而限制其应用范围.本文通过理论模型分析和实验测量,验证了温度、磁场、螺旋冷阱材质等对冷冻恢复过程中超极化<sup>129</sup>Xe弛豫的影响;同时,测量了自动收集-升华装置的稳定性.研究结果表明,升华方式和冷阱材质对<sup>129</sup>Xe极化度损耗的影响显著;自制收集-升华装置的自动化程度高、长时间稳定,<sup>129</sup>Xe极化度的恢复率可达到85.6% ± 4.7%.本研究非常有助于提升超极化<sup>129</sup>Xe在动物和人体MRI中的使用效率.
129Xe nuclear magnetic resonance study of xenon adsorbed on zeolite NaY exchanged with alkali-metal and alkaline-earth cations
[J].
Measuring nanopore size from the spin-lattice relaxation of CF4 gas
[J].The NMR 19F spin-lattice relaxation time constant T1 for CF4 gas is dominated by spin-rotation interaction, which is mediated by the molecular collision frequency. When confined to pores of approximately the same size or smaller than the bulk gas mean free path, additional collisions of molecules with the pore walls should substantially change T1. To develop a method for measuring the surface/volume ratio S/V by measuring how T1 changes with confinement, we prepared samples of known S/V from fumed silica of known mass-specific surface area and compressed to varying degrees into cylinders of known volume. We then measured T1 for CF4 in these samples at varying pressures, and developed mathematical models for the change in T1 to fit the data. Even though CF4 has a critical temperature below room temperature, we found that its density in pores was greater than that of the bulk gas and that it was necessary to take this absorption into account. We modeled adsorption in two ways, by assuming that the gas condenses on the pore walls, and by assuming that gas in a region near the wall is denser than the bulk gas because of a simplified attractive potential. Both models suggested the same two-parameter formula, to which we added a third parameter to successfully fit the data and thus achieved a rapid, precise way to measure S/V from the increase in T1 due to confinement in pores.
NMR methods for characterizing the pore structures and hydrogen storage properties of microporous carbons
[J].
DOI:10.1021/ja9109924
PMID:20524615
[本文引用: 1]

(1)H NMR spectroscopy is used to investigate a series of microporous activated carbons derived from a poly(ether ether ketone) (PEEK) precursor with varying amounts of burnoff (BO). In particular, properties relevant to hydrogen storage are evaluated such as pore structure, average pore size, uptake, and binding energy. High-pressure NMR with in situ H(2) loading is employed with H(2) pressure ranging from 100 Pa to 10 MPa. An N(2)-cooled cryostat allows for NMR isotherm measurements at both room temperature ( approximately 290 K) and 100 K. Two distinct (1)H NMR peaks appear in the spectra which represent the gaseous H(2) in intergranular pores and the H(2) residing in micropores. The chemical shift of the micropore peak is observed to evolve with changing pressure, the magnitude of this effect being correlated to the amount of BO and therefore the structure. This is attributed to the different pressure dependence of the amount of adsorbed and non-adsorbed molecules within micropores, which experience significantly different chemical shifts due to the strong distance dependence of the ring current effect. In pores with a critical diameter of 1.2 nm or less, no pressure dependence is observed because they are not wide enough to host non-adsorbed molecules; this is the case for samples with less than 35% BO. The largest estimated pore size that can contribute to the micropore peak is estimated to be around 2.4 nm. The total H(2) uptake associated with pores of this size or smaller is evaluated via a calibration of the isotherms, with the highest amount being observed at 59% BO. Two binding energies are present in the micropores, with the lower, more dominant one being on the order of 5 kJ mol(-1) and the higher one ranging from 7 to 9 kJ mol(-1).
Hydrogen storage and mobility determined by NMR to various organically functionalized porous silica synthetized by using the post-grafting method
[J].
Determination of chemical shift of gas-phase hydrogen molecules by 1H nuclear magnetic resonance
[J].
Determination of apparent protein molecular weight in solution by diffusion ordered NMR spectroscopy
[J].
扩散序谱 (DOSY) 实验测定缓冲体系中蛋白质表观分子量
[J].
DOI:10.11938/cjmr20182625
[本文引用: 1]

液体核磁共振扩散序谱(DOSY)可以通过测定溶质分子的自扩散系数(D<sub>t</sub>)来研究该分子在溶液中的表观分子量(M).D<sub>t</sub>与测试体系和分子本身性质相关,蛋白质体系较为复杂,从而增加了蛋白质自扩散系数(D<sub>t-protein</sub>)测定的难度.本文以3-(三甲基硅基)丙磺酸钠(DSS)为内标,以蛋白质分子与DSS自扩散系数的比值(D<sub>r</sub>)来表征蛋白质分子在溶液中的表观分子量(M<sub>protein</sub>),该方法降低了缓冲体系对M<sub>protein</sub>的影响,使得M<sub>protein</sub>主要由分子本身的性质决定.在此基础上,测定了不同分子量蛋白质分子相对于DSS的D<sub>r</sub>,拟合得到了D<sub>r</sub>与M<sub>protein</sub>的相关关系:lgM<sub>protein</sub> =-2.6488 lgD<sub>r</sub>-0.7863,相关系数(R<sup>2</sup>)为0.997.最后测定了通过大肠杆菌表达纯化得到的SARS冠状病毒主蛋白酶C端结构域(M<sup>pro</sup>-C)分子相对于DSS的D<sub>r</sub>,并计算出与文献结果一致的M<sub>protein</sub>,进一步验证了拟合公式的准确性和实用性.
Effect of pore size on the adsorption of xenon on mesoporous MCM-41 and on the 129Xe NMR chemical shifts: A variable temperature study
[J].
Variable temperature 129Xe NMR studies of xenon adsorbed on mesoporous MCM-41 molecular sieves
[J].
Tutorial: a beginner’s guide to interpreting magnetic susceptibility data with the Curie-Weiss law
[J].
Magnetic resonance characterization of porous media using diffusion through internal magnetic fields
[J].
DOI:10.3390/ma5040590
PMID:28816998
[本文引用: 1]

When a porous material is inserted into a uniform magnetic field, spatially varying fields typically arise inside the pore space due to susceptibility contrast between the solid matrix and the surrounding fluid. As a result, direct measurement of the field variation may provide a unique opportunity to characterize the pore geometry. The sensitivity of nuclear magnetic resonance (NMR) to inhomogeneous field variations through their dephasing effects on diffusing spins is unique and powerful. Recent theoretical and experimental research sheds new light on how to utilize susceptibility-induced internal field gradients to quantitatively probe the microstructure of porous materials. This article reviews ongoing developments based on the stimulated echo-pulse sequence to extend the characterization of porous media using both spatially resolved and unresolved susceptibility-induced internal gradients that operate on a diffusing-spin ensemble.
Nuclear magnetic resonance of 3He dissolved in vitreous silica
[J].