
- Jul. 12, 2025
- Home
- About Us
- Editorial Board
- Instruction
- Subscription
- Advertisement
- Contact Us
- Chinese
- RSS

Chinese Journal of Magnetic Resonance ›› 2023, Vol. 40 ›› Issue (4): 385-396.doi: 10.11938/cjmr20233062
• Articles • Previous Articles Next Articles
CHEN Mengying,WU Yupeng,PANG Qifan,ZHONG Haodong,LI Gaiying,LI Jianqi*( )
)
Received:2023-03-28
Published:2023-12-05
Online:2023-05-16
CLC Number:
CHEN Mengying, WU Yupeng, PANG Qifan, ZHONG Haodong, LI Gaiying, LI Jianqi. Simultaneously Neuromelanin-sensitive Imaging and Quantitative Susceptibility Mapping in the Whole Brain[J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 385-396.

Fig. 1
A schematic diagram of the 3D MT-GRE sequence. The sequence consists of an MT module and a 3D GRE imaging module. In the MT module, a spoiler gradient (black) is added following the Gaussian RF pulse (green) to spoil residual transverse magnetization. In the GRE module, a strong spoiler gradient (black) is applied to eliminate the residual transverse magnetization after all echoes are collected. α, flip angle for GRE imaging module; TR, repetition time; TE1, the first echo time; ΔTE, echo spacing


Fig. 2
Regions of interest (ROIs) for quantitative analysis of tissue contrast in neuromelanin (NM) sensitive images and susceptibility values in susceptibility maps. (a) ROIs for NM analysis. Red and orange circles were drawn for substantia nigra and reference areas, respectively. (b)~(d) ROIs for susceptibility analysis. CN, caudate nucleus; PUT, putamen; GP, globus pallidus; RN, red nucleus; SN, substantia nigra; DN, dentate nucleus
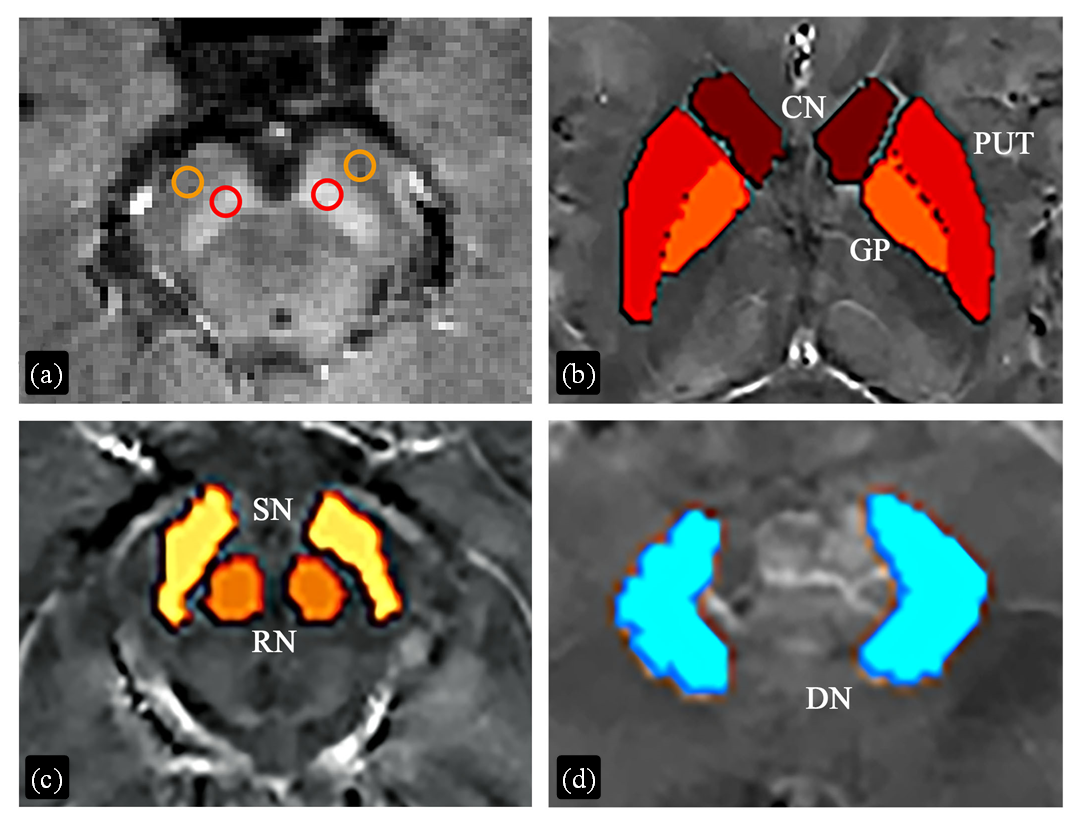
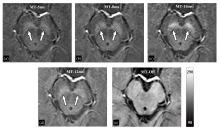
Fig. 3
The magnitude images of the first echo acquired by 3D GRE sequences with or without MT pulse. (a) MT RF pulse lasted for 5 ms (MT-5ms); (b) MT RF pulse lasted for 8 ms (MT-8ms); (c) MT RF pulse lasted for 10 ms (MT-10ms); (d) MT RF pulse lasted for 12 ms (MT-12ms); (e) MT RF pulse was not applied (MT-Off)

Table 1
Comparison of magnetic susceptibility values in deep gray matter nuclei acquired with and without MT pulses
| 核团 | 磁化率值( | 配对样本t检验(p值) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MT-5ms | MT-8ms | MT-10ms | MT-12ms | MT-Off | MT-5ms vs. MT-Off | MT-8ms vs. MT-Off | MT-10ms vs. MT-Off | MT-12ms vs. MT-Off | ||
| 尾状核 | 53±13 | 55±13 | 55±13 | 51±13 | 52±12 | 0.450 | 0.130 | 0.145 | 0.967 | |
| 壳核 | 38±10 | 39±13 | 38±11 | 36±10 | 36±11 | 0.485 | 0.221 | 0.469 | 0.971 | |
| 苍白球 | 127±13 | 125±11 | 126±13 | 123±13 | 127±10 | 0.738 | 0.227 | 0.450 | 0.288 | |
| 红核 | 67±21 | 66±18 | 64±17 | 65±22 | 63±21 | 0.210 | 0.196 | 0.845 | 0.618 | |
| 黑质 | 81±13 | 80±10 | 79±10 | 78±13 | 79±12 | 0.278 | 0.490 | 0.947 | 0.757 | |
| 齿状核 | 74±11 | 73±9 | 74±12 | 71±10 | 73±10 | 0.706 | 0.879 | 0.378 | 0.489 | |

Fig. 5
Quantitative comparison of the susceptibility values acquired with MT pulses of different durations and without MT pulse. (a), (c), (e), (g) scattered plots of the linear regression analysis of susceptibility values. The solid and dotted lines are the trend line of the linear regression and the line of equality, respectively. (b), (d), (f), (h) Bland-Altman plots. The solid and dotted lines indicate the mean difference and the mean difference ± 1.96 times the standard deviation of the difference, respectively. CN, caudate nucleus; PUT, putamen; GP, globus pallidus; RN, red nucleus; SN, substantia nigra; DN, dentate nucleus

| [1] |
OHTSUKA C, SASAKI M, KONNO K, et al. Changes in substantia nigra and locus coeruleus in patients with early-stage Parkinson’s disease using neuromelanin-sensitive MR imaging[J]. Neurosci Lett, 2013, 541: 93-98.
doi: 10.1016/j.neulet.2013.02.012 |
| [2] |
ZUCCA F A, SEGURA-AGUILAR J, FERRARI E, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease[J]. Prog Neurobiol, 2017, 155: 96-119.
doi: 10.1016/j.pneurobio.2015.09.012 |
| [3] |
ZECCA L, BELLEI C, COSTI P, et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals[J]. Proc Natl Acad Sci USA, 2008, 105(45): 17567-17572.
doi: 10.1073/pnas.0808768105 pmid: 18988735 |
| [4] |
MOSHAROV E V, LARSEN K E, KANTER E, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons[J]. Neuron, 2009, 62(2): 218-229.
doi: 10.1016/j.neuron.2009.01.033 pmid: 19409267 |
| [5] |
TRUJILLO P, SUMMERS P E, FERRARI E, et al. Contrast mechanisms associated with neuromelanin-MRI[J]. Magn Reson Med, 2017, 78(5): 1790-1800.
doi: 10.1002/mrm.26584 pmid: 28019018 |
| [6] |
SASAKI M, SHIBATA E, TOHYAMA K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease[J]. Neuroreport, 2006, 17(11): 1215-1218.
doi: 10.1097/01.wnr.0000227984.84927.a7 |
| [7] |
RENEMAN L, VAN DER PLUIJM M, SCHRANTEE A, et al. Imaging of the dopamine system with focus on pharmacological MRI and neuromelanin imaging[J]. Eur J Radiol, 2021, 140: 109752.
doi: 10.1016/j.ejrad.2021.109752 |
| [8] |
LANGLEY J, HUDDLESTON D E, LIU C J, et al. Reproducibility of locus coeruleus and substantia nigra imaging with neuromelanin sensitive MRI[J]. Magn Reson Mat Phys Biol Med, 2016, 30(2): 121-125.
doi: 10.1007/s10334-016-0590-z |
| [9] | YANG J Q, YANG X F, ZHONG C Q, et al. The application value of neuromelanin sensitive magnetic resonance imaging in the diagnosis of Parkinson’s disease[J] J Clin Radiol, 2021, 40(5): 5. |
| 杨俊强, 杨晓帆, 仲崇琦, 等. 神经黑色素敏感磁共振成像对诊断帕金森病的应用价值[J]. 临床放射学杂志, 2021, 40(5): 5. | |
| [10] |
MATSUURA K, MAEDA M, TABEI K I, et al. A longitudinal study of neuromelanin-sensitive magnetic resonance imaging in Parkinson’s disease[J]. Neurosci Lett, 2016, 633: 112-117.
doi: 10.1016/j.neulet.2016.09.011 |
| [11] |
XIANG Y, GONG T, WU J, et al. Subtypes evaluation of motor dysfunction in Parkinson’s disease using neuromelanin-sensitive magnetic resonance imaging[J]. Neurosci Lett, 2017, 638: 145-150.
doi: 10.1016/j.neulet.2016.12.036 |
| [12] |
WANG J, HUANG Z, LI Y, et al. Neuromelanin-sensitive MRI of the substantia nigra: an imaging biomarker to differentiate essential tremor from tremor-dominant Parkinson’s disease[J]. Parkinsonism Relat Disord, 2019, 58: 3-8.
doi: 10.1016/j.parkreldis.2018.07.007 |
| [13] | LEITAO R, GUERREIRO C, NUNES R G, et al. Neuromelanin magnetic resonance imaging of the substantia nigra in Huntington’s disease[J]. J Huntingtons Dis, 2020, 9(2): 143-148. |
| [14] |
SHIBATA E, SASAKI M, TOHYAMA K, et al. Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus[J]. Biol Psychiatry, 2008, 64(5): 401-406.
doi: 10.1016/j.biopsych.2008.03.021 |
| [15] |
CASSIDY C M, CARPENTER K M, KONOVA A B, et al. Evidence for dopamine abnormalities in the substantia nigra in cocaine addiction revealed by neuromelanin-sensitive MRI[J]. Am J Psychiatry, 2020, 177(11): 1038-1047.
doi: 10.1176/appi.ajp.2020.20010090 |
| [16] |
DE ROCHEFORT L, LIU T, KRESSLER B, et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: validation and application to brain imaging[J]. Magn Reson Med, 2010, 63(1): 194-206.
doi: 10.1002/mrm.22187 pmid: 19953507 |
| [17] |
LANGKAMMER C, SCHWESER F, KREBS N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study[J]. NeuroImage, 2012, 62(3): 1593-1599.
doi: 10.1016/j.neuroimage.2012.05.049 pmid: 22634862 |
| [18] |
LI G, WU R, TONG R, et al. Quantitative measurement of metal accumulation in brain of patients with Wilson’s disease[J]. Mov Disord, 2020, 35(10): 1787-1795.
doi: 10.1002/mds.v35.10 |
| [19] |
BULK M, ABDELMOULA W M, GEUT H, et al. Quantitative MRI and laser ablation-inductively coupled plasma-mass spectrometry imaging of iron in the frontal cortex of healthy controls and Alzheimer’s disease patients[J]. Neuroimage, 2020, 215: 116808.
doi: 10.1016/j.neuroimage.2020.116808 |
| [20] |
HUANG W, SWEENEY E M, KAUNZNER U W, et al. Quantitative susceptibility mapping versus phase imaging to identify multiple sclerosis iron rim lesions with demyelination[J]. J Neuroimaging, 2022, 32(4): 667-675.
doi: 10.1111/jon.12987 pmid: 35262241 |
| [21] |
WANG S, LOU M, LIU T, et al. Hematoma volume measurement in gradient echo MRI using quantitative susceptibility mapping[J]. Stroke, 2013, 44(8): 2315-2317.
doi: 10.1161/STROKEAHA.113.001638 pmid: 23704111 |
| [22] |
CHEN W, ZHU W, KOVANLIKAYA I, et al. Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping[J]. Radiology, 2014, 270(2): 496-505.
doi: 10.1148/radiol.13122640 pmid: 24126366 |
| [23] |
AZUMA M, HIRAI T, YAMADA K, et al. Lateral asymmetry and spatial difference of iron deposition in the substantia nigra of patients with Parkinson disease measured with quantitative susceptibility mapping[J]. Am J Neuroradiol, 2016, 37(5): 782-788.
doi: 10.3174/ajnr.A4645 pmid: 26822728 |
| [24] |
AN H, ZENG X, NIU T, et al. Quantifying iron deposition within the substantia nigra of Parkinson’s disease by quantitative susceptibility mapping[J]. J Neurol Sci, 2018, 386: 46-52.
doi: 10.1016/j.jns.2018.01.008 |
| [25] |
BERGSLAND N, ZIVADINOV R, SCHWESER F, et al. Ventral posterior substantia nigra iron increases over 3 years in Parkinson’s disease[J]. Mov Disord, 2019, 34(7): 1006-1013.
doi: 10.1002/mds.v34.7 |
| [26] |
SJOSTROM H, GRANBERG T, WESTMAN E, et al. Quantitative susceptibility mapping differentiates between parkinsonian disorders[J]. Parkinsonism Relat Disord, 2017, 44: 51-57.
doi: 10.1016/j.parkreldis.2017.08.029 |
| [27] | WU M Z, LUAN J X, ZHANG C C, et al. Meta analysis of quantitative susceptibility mapping of substantia nigra in the diagnosis of Parkinson’s disease[J] Chin J Magn Reson Imaging, 2023, 14(2): 6-11. |
| 吴明振, 栾继昕, 张传臣, 等. 黑质定量磁化率成像对帕金森病诊断价值的Meta分析[J]. 磁共振成像, 2023, 14(2): 6-11. | |
| [28] | TAKAHASHI H, WATANABE Y, TANAKA H, et al. Quantifying changes in nigrosomes using quantitative susceptibility mapping and neuromelanin imaging for the diagnosis of early-stage Parkinson’s disease[J]. Br J Radiol, 2018, 91(1086): 20180037. |
| [29] |
TAKAHASHI H, WATANABE Y, TANAKA H, et al. Comprehensive MRI quantification of the substantia nigra pars compacta in Parkinson’s disease[J]. Eur J Radiol, 2018, 109: 48-56.
doi: 10.1016/j.ejrad.2018.06.024 |
| [30] |
WANG X, ZHANG Y, ZHU C, et al. The diagnostic value of SNpc using NM-MRI in Parkinson’s disease: meta-analysis[J]. Neurol Sci, 2019, 40(12): 2479-2489.
doi: 10.1007/s10072-019-04014-y |
| [31] |
HE N, GHASSABAN K, HUANG P, et al. Imaging iron and neuromelanin simultaneously using a single 3D gradient echo magnetization transfer sequence: Combining neuromelanin, iron and the nigrosome-1 sign as complementary imaging biomarkers in early stage Parkinson’s disease[J]. NeuroImage, 2021, 230: 117810.
doi: 10.1016/j.neuroimage.2021.117810 |
| [32] |
JIN L, WANG J, WANG C, et al. Combined visualization of nigrosome-1 and neuromelanin in the substantia nigra using 3T MRI for the differential diagnosis of essential tremor and de novo Parkinson’s disease[J]. Front Neurol, 2019, 10: 100.
doi: 10.3389/fneur.2019.00100 |
| [33] |
ARMSTRONG M J, OKUN M S. Diagnosis and treatment of Parkinson disease: a review[J]. J Am Med Assoc, 2020, 323(6): 548-560.
doi: 10.1001/jama.2019.22360 |
| [34] |
WANG J Y, ZHUANG Q Q, ZHU L B, et al. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements[J]. Sci Rep, 2016, 6: 36669.
doi: 10.1038/srep36669 |
| [35] |
KARSA A, PUNWANI S, SHMUELI K. The effect of low resolution and coverage on the accuracy of susceptibility mapping[J]. Magn Reson Med, 2019, 81(3): 1833-1848.
doi: 10.1002/mrm.27542 pmid: 30338864 |
| [36] |
HENKELMAN R M, STANISZ G J, GRAHAM S J. Magnetization transfer in MRI: a review[J]. NMR Biomed, 2001, 14(2): 57-64.
pmid: 11320533 |
| [37] |
WENGLER K, CASSIDY C, VAN DER PLUIJM M, et al. Cross-scanner harmonization of neuromelanin-sensitive MRI for multisite studies[J]. J Magn Reson Imaging, 2021, 54(4): 1189-1199.
doi: 10.1002/jmri.27679 pmid: 33960063 |
| [38] | BERNSTEIN M A, KING K F, ZHOU X J. Handbook of MRI pulse sequences[M]. Burlington: Academic Press, 2004: 96-124. |
| [39] |
PIKE G B, GLOVER G H, HU B S, et al. Pulsed magnetization transfer spin-echo MR imaging[J]. J Magn Reson Imaging, 1993, 3(3): 531-539.
pmid: 8324313 |
| [40] |
BIONDETTI E, KARSA A, THOMAS D L, et al. Investigating the accuracy and precision of TE-dependent versus multi-echo QSM using laplacian-based methods at 3 T[J]. Magn Reson Med, 2020, 84(6): 3040-3053.
doi: 10.1002/mrm.v84.6 |
| [41] |
SMITH S M. Fast robust automated brain extraction[J]. Hum Brain Mapp, 2002, 17(3): 143-155.
doi: 10.1002/hbm.10062 pmid: 12391568 |
| [42] |
LIU T, WISNIEFF C, LOU M, et al. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping[J]. Magn Reson Med, 2013, 69(2): 467-476.
doi: 10.1002/mrm.24272 pmid: 22488774 |
| [43] | ZHAO X X, BO B S, LIU T, et al. Research on multi-echo phase fitting algorithm for quantitative susceptibility mapping[J]. Chinese J Magn Reson, 2016, 33(4): 609-617. |
| 赵欣欣, 薄斌仕, 刘田, 等. 定量磁化率成像多回波相位拟合算法研究[J]. 波谱学杂志, 2016, 33(4): 609-617. | |
| [44] |
ABDUL-RAHMAN H S, GDEISAT M A, BURTON D R, et al. Fast and robust three-dimensional best path phase unwrapping algorithm[J]. Appl Opt, 2007, 46(26): 6623-6635.
doi: 10.1364/AO.46.006623 |
| [45] |
ZHOU D, LIU T, SPINCEMAILLE P, et al. Background field removal by solving the Laplacian boundary value problem[J]. NMR Biomed, 2014, 27(3): 312-319.
doi: 10.1002/nbm.3064 pmid: 24395595 |
| [46] |
LIU Z, SPINCEMAILLE P, YAO Y, et al. MEDI+0: morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping[J]. Magn Reson Med, 2018, 79(5): 2795-2803.
doi: 10.1002/mrm.26946 pmid: 29023982 |
| [47] |
LIU J, LIU T, DE ROCHEFORT L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map[J]. NeuroImage, 2012, 59(3): 2560-2568.
doi: 10.1016/j.neuroimage.2011.08.082 pmid: 21925276 |
| [48] |
LIU T, XU W, SPINCEMAILLE P, et al. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI[J]. IEEE Trans Med Imaging, 2012, 31(3): 816-824.
doi: 10.1109/TMI.2011.2182523 |
| [49] |
SCHWARZ S T, RITTMAN T, GONTU V, et al. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson’s disease[J]. Mov Disord, 2011, 26(9): 1633-1638.
doi: 10.1002/mds.23722 |
| [50] |
OGISU K, KUDO K, SASAKI M, et al. 3D neuromelanin-sensitive magnetic resonance imaging with semi-automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson’s disease[J]. Neuroradiology, 2013, 55(6): 719-724.
doi: 10.1007/s00234-013-1171-8 |
| [51] |
CHEN X, HUDDLESTON D E, LANGLEY J, et al. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach[J]. Magn Reson Imaging, 2014, 32(10): 1301-1306.
doi: 10.1016/j.mri.2014.07.003 pmid: 25086330 |
| [52] |
DIMOV A V, GUPTA A, KOPELL B H, et al. High-resolution QSM for functional and structural depiction of subthalamic nuclei in DBS presurgical mapping[J]. J Neurosurg, 2018, 131(2): 360-367.
doi: 10.3171/2018.3.JNS172145 pmid: 30095333 |
| [53] |
VITEK J L, LYONS K E, BAKAY R, et al. Standard guidelines for publication of deep brain stimulation studies in Parkinson’s disease (Guide4DBS-PD)[J]. Mov Disord, 2010, 25(11): 1530-1537.
doi: 10.1002/mds.v25:11 |
| [54] |
ASHKAN K, BLOMSTEDT P, ZRINZO L, et al. Variability of the subthalamic nucleus: the case for direct MRI guided targeting[J]. Br J Neurosurg, 2007, 21(2): 197-200.
pmid: 17453788 |
| [55] |
LIU T, ESKREIS-WINKLER S, SCHWEITZER A D, et al. Improved subthalamic nucleus depiction with quantitative susceptibility mapping[J]. Radiology, 2013, 269(1): 216-223.
doi: 10.1148/radiol.13121991 pmid: 23674786 |
| [56] |
ZHAO W, WANG Y, ZHOU F, et al. Automated segmentation of midbrain structures in high-resolution susceptibility maps based on convolutional neural network and transfer learning[J]. Front Neurosci, 2022, 16: 801618.
doi: 10.3389/fnins.2022.801618 |
| [57] |
HUA J, HURST G C. Analysis of on- and off-resonance magnetization transfer techniques[J]. J Magn Reson Imaging, 1995, 5(1): 113-120.
pmid: 7696801 |
| [58] | BAOGUI Z, KUN W, TIANZI J. RF power design optimization in MRI system[J]. Magn Reson Lett, 2021, 1(1): 89-98. |
| [1] | CUI Jiaqi, LIU Kangqi, LI Junyao, WANG Fang, MING Gang, ZHAO Feng, MEI Ganghua, ZHONG Da. Investigation of an Ultra High Signal-to-noise Ratio Physics Package for the Rubidium Atomic Clock [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 462-470. |
| [2] | SONG Linhong, CHAI Xin, ZHANG Xu, JIANG Bin, LIU Maili. Optimizing Sensitivity-enhanced Quantitative 13C NMR Experiment by Genetic Algorithm [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 365-375. |
| [3] | ZHOU Tianli, ZHANG Dian, WU Jizhi, JIA Huihui, CHANG Yan, SHENG Mao, YANG Xiaodong. Application of MRI-based Finite Element Modeling and Analysis in Periacetabular Osteotomy [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 397-409. |
| [4] | SHEN Changzhi, ZHANG Linlin, LI Xin, SHEN Wanling. Hydrothermal Crystallization of Niobium Oxide Nanorods Studied by 93Nb Nuclear Magnetic Resonance [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 376-384. |
| [5] | LI Ren, CHANG Xiao, ZHANG Jie, ZHANG Xiaoyong. Progress of Magnetic Resonance Spectroscopy in the Study of the Effects of Smoking on the Brain [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 471-480. |
| [6] | ZHAO Wanlei, ZHAO Zhihong, ZHANG Minghui, LIU Wenjing. Study on Moisture Absorption and Water Absorption of PMMA Wood-plastic Composites Based on TD-NMR [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 448-461. |
| [7] | REN Hongjin, MA Yan, XIAO Liang. Knee Joint Model Construction and Local Specific Absorption Rate Estimation Based on Generative Adversarial Networks [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 410-422. |
| [8] | LAI Jiawen, WANG Yuling, CAI Xiaoyu, ZHOU Lihua. Multidimensional Information Fusion Method for Meniscal Tear Classification Based on CNN-SVM [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 423-434. |
| [9] | WANG Hui, WANG Tiantian, WANG Lijia. Squeeze-and-excitation Residual U-shaped Network for Left Myocardium Segmentation Based on Cine Cardiac Magnetic Resonance Images [J]. Chinese Journal of Magnetic Resonance, 2023, 40(4): 435-447. |
| [10] | ZHANG Haowei , WANG Yuncheng , LIU Ying. Brain Age Assessment of Major Depressive Disorder patients Based on Convolutional Neural Network [J]. Chinese Journal of Magnetic Resonance, 0, (): 0-0. |
| [11] | ZHOU Minxiong , QI Xuan , DU Bin , QI Dong, NIE Shengdong, WANG Haijie, YANG Guang, LIU Mengxiao, ZHANG Huiting, Cai Wenmei, YAN Xu, HE Yongsheng. Evaluation of the Impact of b-Value Ranges on Six Body Diffusion Models in Prostate Application [J]. Chinese Journal of Magnetic Resonance, 0, (): 0-0. |
| [12] | Ma Huifang , Tong Yue , Wang Rongfan, Xie Jianwei . Discovery and Structural Characterization of Impurities in the Synthesis of Darolutamide Intermediate [J]. Chinese Journal of Magnetic Resonance, 0, (): 0-0. |
| [13] | Dai Junlong , He Cong , Wu Jie , Bian Yun , #. Pancreatic Cystic Neoplasms Segmentation Network Combining Dual Decoding and Global Attention Upsampling Modules [J]. Chinese Journal of Magnetic Resonance, 0, (): 0-0. |
| [14] | CHEN Yang, ZHOU Meng, LI Yong, YANG Haijun. Independent Development of Sample Preparation Tools for Solid-State NMR [J]. Chinese Journal of Magnetic Resonance, 0, (): 0-0. |
| [15] | HU Xiaoyang, LIU Ying, CHEN Shu, DONG Binbin. Fusing Attention Mechanism with Mask RCNN for Recognition of Acoustic Neuroma and Meningioma in Cerebellopontine Angle [J]. Chinese Journal of Magnetic Resonance, 2023, 40(3): 293-306. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 219
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 150
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||