摘要: α-对取代苯基异戊酸是合成拟除虫菊酯的重要中间化合物,其结构中有手性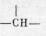 和前手性两个甲基。为了解构效与活性的关系,本文对其结构特点进行了仔细的考察。用手性位移试剂Eu(hfbc)3和位移试剂Eu(fod)3-分离了对映体的1H和13C的信号,并作出了一定的解释,为鉴定化合物的光学纯度找出了一种简便的方法。对异丙基上的两个非对映异位甲基作了详细的观察,确定了优势构象,并对其作了标识,实验与由简单模型的计算结果一致。本文认为在对这两个非对映异位甲基信号位移差别大小的影响中,取代基的体积效应起了相当重要的作用。
和前手性两个甲基。为了解构效与活性的关系,本文对其结构特点进行了仔细的考察。用手性位移试剂Eu(hfbc)3和位移试剂Eu(fod)3-分离了对映体的1H和13C的信号,并作出了一定的解释,为鉴定化合物的光学纯度找出了一种简便的方法。对异丙基上的两个非对映异位甲基作了详细的观察,确定了优势构象,并对其作了标识,实验与由简单模型的计算结果一致。本文认为在对这两个非对映异位甲基信号位移差别大小的影响中,取代基的体积效应起了相当重要的作用。
焦钉, 沈其丰. 拟除虫菊酯类农药的NMR研究Ⅰ.α-对取代苯基异戊酸及其类似化合物的手性和前手性NMR研究[J]. 波谱学杂志, 1988, 5(3): 233-244.
Jiao Ding, Shen Qifeng. NMR STUDIES ON SYNTHETIC PESTICIDES I. NMR STUDIES ON CHIRALITY AND PROCHIALITY OF α (P-CI-PHENYL) ISOAMYL ACID AND ITS ANALOQUES[J]. Chinese Journal of Magnetic Resonance, 1988, 5(3): 233-244.

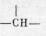 和前手性两个甲基。为了解构效与活性的关系,本文对其结构特点进行了仔细的考察。用手性位移试剂Eu(hfbc)3和位移试剂Eu(fod)3-分离了对映体的1H和13C的信号,并作出了一定的解释,为鉴定化合物的光学纯度找出了一种简便的方法。对异丙基上的两个非对映异位甲基作了详细的观察,确定了优势构象,并对其作了标识,实验与由简单模型的计算结果一致。本文认为在对这两个非对映异位甲基信号位移差别大小的影响中,取代基的体积效应起了相当重要的作用。
和前手性两个甲基。为了解构效与活性的关系,本文对其结构特点进行了仔细的考察。用手性位移试剂Eu(hfbc)3和位移试剂Eu(fod)3-分离了对映体的1H和13C的信号,并作出了一定的解释,为鉴定化合物的光学纯度找出了一种简便的方法。对异丙基上的两个非对映异位甲基作了详细的观察,确定了优势构象,并对其作了标识,实验与由简单模型的计算结果一致。本文认为在对这两个非对映异位甲基信号位移差别大小的影响中,取代基的体积效应起了相当重要的作用。