引言
谷胱甘肽是一种由谷氨酸、甘氨酸和半胱氨酸形成的三肽,具有很强的还原性.在生物活体中,谷胱甘肽分为还原型谷胱甘肽(GSH)和氧化型谷胱甘肽(GSSG),其中还原型谷胱甘肽占绝大多数,是人体主要的还原性驱动力,参与了如自由基淬灭、重金属解毒等生化反应[1,2].谷胱甘肽还与肿瘤治疗密切相关,例如,在放疗中利用活性氧杀伤肿瘤细胞,其效果会因为谷胱甘肽与活性氧发生反应而受到影响,在进行以生成活性氧为手段的肿瘤治疗中,肿瘤附近的谷胱甘肽浓度对于疗效的影响至关重要[3,4].作为人体内重要的解毒剂,谷胱甘肽含量变化能反映人体解毒器官(如肝脏)的功能和状态[5,6],脑部谷胱甘肽含量的下降可能与早期阿尔兹海默症及认知障碍相关[7,8].
作为一种无损伤且非侵入性的方法,磁共振波谱(MRS)在研究活体代谢分子及疾病标记物的含量变化中表现出独特的优越性, 其在临床应用中发展迅速.例如,MRS可以连续观察多种在神经生物学上起重要作用的代谢物的浓度,通过比较脑组织及肿瘤内某些代谢物的浓度变化,实现对病变的代谢成像和定性诊断[9,10].同时,由于生物活体环境十分复杂,器官、组织、体液中含有大量的生物大分子、无机物、有机代谢产物等,常规的1H-MRS谱往往谱峰重叠严重.为了选择性地检测不同分子信号的MEGA-PRESS(MEshcher-GArwood Point RESolved Spectroscopy)技术、多量子滤波等技术应运而生,先后被应用于对γ-氨基丁酸(GABA)、谷氨酸(Glu)、谷胱甘肽、乳酸等内源分子的靶向信号选择[11⇓⇓-14].2009年,Choi等人[15,16]利用双量子滤波方法实现了谷胱甘肽分子的活体MRS信号选择,该方法对传统双量子滤波进行了进一步的优化,通过零量子态和双量子态的相互转化和对双量子态的二次编码,实现对特定分子信号的高效选择.但其也存在一定的不足:用于激发信号的选择性脉冲的时长过长,导致信号衰减严重,当适当减小脉冲时长时,分子的选择性则会变差.近年来,核自旋单重态被应用于活体分子的靶向选择,根据分子的化学参数设计特定的磁共振脉冲,将分子中的核自旋转化为单重态,利用其寿命长、不受梯度场影响[17⇓-19]的特殊性质实现分子信号选择.优化脉冲根据GRAPE(Gradient Ascent Pulse Engineering)算法计算获得[20],计算过程中脉冲的变量多,可编辑性强,单重态的制备效率高.作者所在课题组前期利用优化控制和数值计算脉冲实现了Glu和GABA分子中的单重态制备及活体分子的选择性检测[21,22],并对多自旋体系中单重态的制备方法进行了理论研究[23,24],为其它分子的单重态制备积累了一定经验.
本文进一步将优化脉冲制备单重态及实现分子滤波的方法应用于活体的谷胱甘肽分子,选择谷胱甘肽分子中两个合适的氢原子核自旋对,通过施加优化的射频脉冲,制备核自旋单重态,同时利用梯度脉冲将其它自旋的信号散相,实现谷胱甘肽分子的选择性检测.该方法针对健康人脑进行了实验验证,结果表明设计的脉冲序列能成功地对不同脑区的谷胱甘肽分子进行选择性检测.
1 材料与方法
1.1 脉冲序列
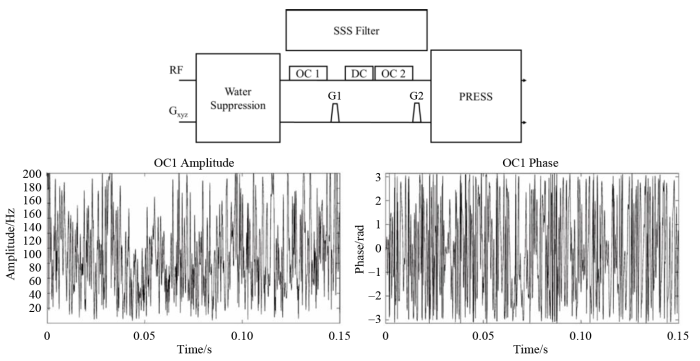
图1展示了利用核自旋单重态实现活体谷胱甘肽分子选择性信号检测的脉冲序列图.其主要思想是在PRESS(Point-Resolved Spectroscopy Sequence)[25]脉冲序列的基础上增加了单重态滤波模块,整个序列由预饱和水信号压制模块、单重态滤波模块和信号采集模块组成.其中单重态滤波模块是实现滤波的关键所在,由两组优化控制脉冲(OC1、OC2)、去耦脉冲(DC)和两组梯度脉冲组成.预饱和脉冲对水信号进行压制之后,OC1脉冲将靶向分子中特定基团制备为核自旋单重态,连续波(CW)去耦脉冲被用于保存单重态,避免其演化为其它状态.利用核自旋单重态不受梯度场影响的特性,使用两组梯度脉冲将非单重态信号散相,实现分子靶向信号选择.核自旋单重态不能直接检测,需要施加第二个优化脉冲OC2将单重态转变为可观测态,最后施加PRESS采集模块进行信号采集.
图1
图1
利用核自旋单重态实现活体谷胱甘肽分子选择性信号检测的脉冲序列图. 其中OC1、OC2分别为制备和转移单重态的优化脉冲模块, 下方两幅波形图为OC1脉冲的功率与相位随时间变化的示意图
Fig. 1
Pulse sequence diagram for selective signal detection of in vivo glutathione molecules using nuclear spin singlet, in which OC1 and OC2 represent the optimized pulse modules for singlet preparation and transferring, respectively. The two waveforms below illustrate the variation of amplitude and phase of the OC1 pulse
1.2 样品与实验参数
实验使用的仪器包括Bruker AVANCE 11.7 T NMR波谱仪和西门子MAGNETOM 3T-Prisma成像仪.
在Bruker AVANCE 11.7 T NMR波谱仪实验中使用的混合水模为肌酸(Cr,北京百灵威科技有限公司,99%)、还原型谷胱甘肽(GSH,MACKLIN,99%,生物技术级)和γ-氨基丁酸(GABA,Aladdin,99%)依次按照物质的量浓度7:3:1的比例混合,溶于重水(damas-beta,99.9%)中,并使用氘代盐酸和氢氧化钠(damas-beta,99.5%)调节pH值为7.2,取600 μL于NMR样品管中.将Cr、GSH、GABA分别溶于重水中,调整pH至7.2,浓度为50 mmol/L,作为单种物质的水模,取600 μL于NMR样品管中,用以测定滤波效率、评估滤波效果.NMR实验温度设置为37 ℃,去耦脉冲功率和时长分别为0.1 W和0.1 s,两段优化脉冲功率和时长均为0.000 3 W和0.07 s,优化脉冲后的散相梯度场强度分别为107.5 mT/m和215.0 mT/m.
在西门子MAGNETOM 3T-Prisma成像仪实验中使用的水模为10 mmol/L GSH水模,称取谷胱甘肽粉末0.76 g溶于去离子水中,使用盐酸和氢氧化钠调节pH至7.2,定容至250 mL.在MRS实验中,TR为1 500 ms,TE为30 ms,采样点数为512,选择的体素大小为20 mm×30 mm×20 mm,去耦脉冲强度为50 Hz,时长为1 000 μs,两个散相梯度场强场度分别为15 mT/m和20 mT/m,为避免产生梯度回波,梯度持续时间1 000 μs.扫描平均次数128次,单次扫描总时长3 min 16 s.
2 OC脉冲理论与计算
优化脉冲在开源软件Simpson平台上实现.一个优化控制脉冲包含2 000个小的脉冲片段,这些小脉冲片段具有不同的功率与相位,通过将脉冲片段依次施加到初始态上,可以以最大的效率实现初始态到目标态的转化.
整个脉冲序列包含两个优化脉冲(OC1和OC2),其中OC1的作用将热平衡态转化为核自旋单重态,OC2则是将核自旋单重态转化为可观测态.GSH分子包含多个氢原子核,选定半胱氨酸(Cys)残基上的亚甲基上的两个氢原子核为研究对象,其初始态为热平衡态,对应的密度算符为:
这里I1、I2代表亚甲基上的两个氢原子核,
3 结果与讨论
3.1 GSH分子靶向滤波目标信号的选取与测试
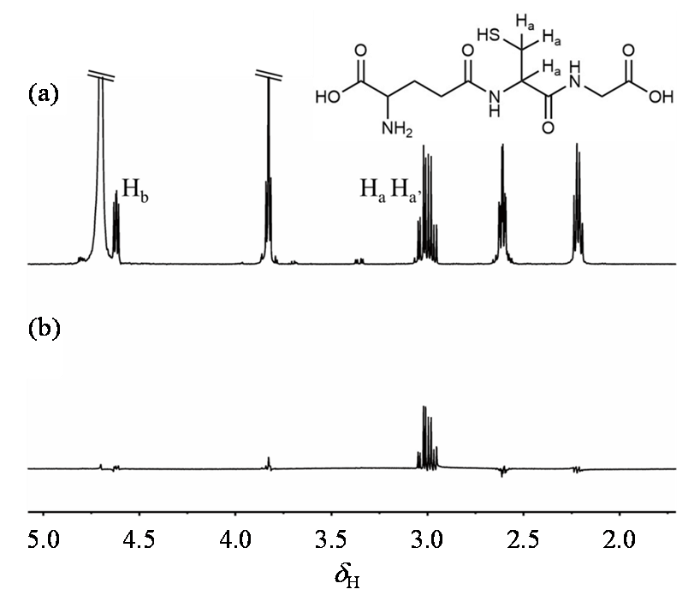
GSH由谷氨酸、半胱氨酸和甘氨酸组成,三个氨基酸分子通过肽键互相连接.选择2.95 ppm位置的半胱氨酸残基亚甲基上的两个氢原子核为研究目标,这也是此前较多GSH分子靶向滤波所选择的目标基团[28]. 半胱氨酸残基上的亚甲基和次甲基的三个质子构成了一个典型的ABX自旋体系.亚甲基上的两个质子不完全等价,且分别受到来自次甲基质子的耦合作用,产生了八重裂分.用于制备单重态的两个氢原子δ = 0.05 ppm,J = -14.14 Hz.利用NMR测量水模中分子的化学位移和J耦合常数,编译Simpson软件进行优化控制脉冲的计算.将计算生成的脉冲应用于水模中,得到的谱图如图2所示.分析实验谱图可知,在应用GSH-SFOC方法滤波后,除目标基团外,其他信号几乎被完全抑制.这表明GSH-SFOC对GSH Cys-CH2的信号具有较好的选择效果,与单脉冲谱图的信号强度对比,测得滤波后的效率约为50%.
图2
图2
在Bruker AVANCE 11.7 T NMR波谱仪平台采集的GSH分子水模及滤波后的1H NMR谱图. (a)使用90˚单脉冲采集到的GSH 1H NMR谱;(b) GSH-SFOC方法滤波后的GSH 1H NMR谱
Fig. 2
1H NMR spectrum and filtered spectrum of GSH molecular phantom acquired on the Bruker AVANCE 11.7 T NMR spectrometer. (a) GSH 1H NMR spectrum collected by using a 90˚single pulse; (b) GSH 1H NMR spectrum after filtering using the GSH-SFOC method
3.2 混合水模GSH分子靶向滤波
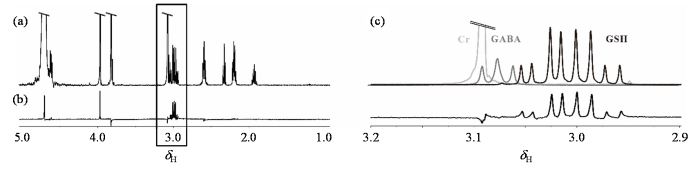
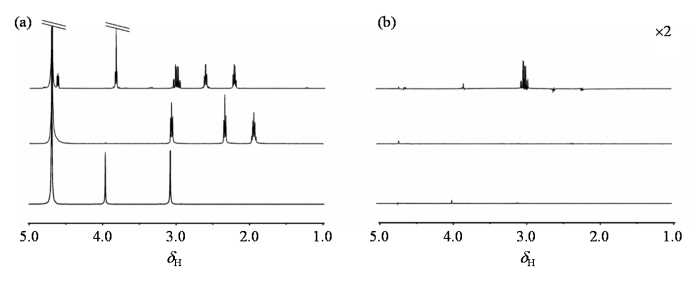
在活体人脑中,GSH分子中基团Cys-CH2与GABA、Cr的氢质子化学位移相近.为了验证滤波方法在活体条件下的可行性,本文配置了三种分子的混合水模,进一步验证GSH-SFOC滤波方法的有效性.实验在Bruker AVANCE 11.7 T NMR波谱仪上完成,结果如图3所示.在常规1H NMR谱[图3(a)和图3(c)]中可以观察到,3.00 ppm附近存在GABA三重峰,GSH八重峰和Cr单峰,三种信号紧密相邻,甚至存在部分重叠.可以预见,在主磁场强度和均匀度相对较低的3 T磁共振成像仪中,随着信号的展宽,重叠会更加严重.Cr的单峰将掩盖GSH的信号,而GABA的信号也会被包含其中.对混合体系施加GSH-SFOC滤波方法,滤波后的1H NMR谱图如图3(b)所示,位于3.03 ppm的Cr信号和3.00 ppm附近的GABA信号几乎完全消失,而3.00 ppm的GSH信号依然清晰可辨,实验结果证明在接近人体环境的混合水模中,GSH-SFOC方法可以有效地滤除Cr、GABA等干扰信号,同时保留GSH中Cys-CH2的信号.
图3
图3
在Bruker AVANCE 11.7 T NMR波谱仪平台采集的混合物水模的1H NMR谱. (a) Cr-GABA-GSH混合物水模的90˚单脉冲1H NMR谱图;(b)对混合物施加GSH-SFOC滤波脉冲后采集的1H NMR谱图;(c)为(a)、(b)中2.9 ppm~3.2 ppm区间的放大图
Fig. 3
1H NMR spectrum and filtered spectrum of Cr-GABA-GSH mixture phantom acquired on the Bruker AVANCE 11.7 T NMR spectrometer. (a) 1H NMR spectrum of the Cr-GABA-GSH mixture phantom using a 90˚ single pulse; (b) 1H NMR spectrum collected after applying GSH-SFOC filtering pulses to the mixture; (c) Enlarged view of the 2.9 ppm~3.2 ppm range of (a) and (b)
图4
图4
在Bruker AVANCE 11.7 T NMR波谱仪平台采集的GSH、Cr和GABA水模的1H NMR谱. (a)从上至下依次为GSH、Cr和GABA的水模对应的90˚单脉冲1H NMR谱图;(b)施加GSH-SFOC脉冲后采集的1H NMR谱图
Fig. 4
1H NMR spectra for GSH, Cr, and GABA phantoms acquired on the Bruker AVANCE 11.7 T NMR spectrometer platform. (a) The 90˚ single-pulse 1H NMR spectra corresponding to the GSH, Cr and GABA phantoms (from top to bottom); (b) The 1H NMR spectra acquired after applying the GSH-SFOC pulse
3.3 活体GSH分子靶向滤波
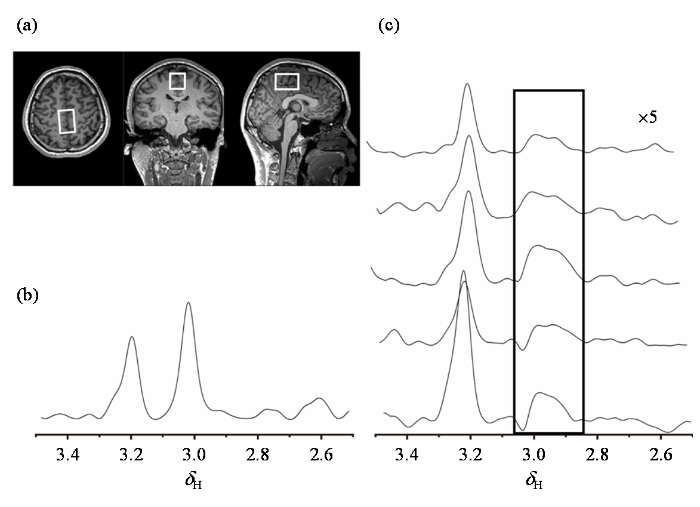
GSH-SFOC脉冲的滤波效果进一步在人体大脑中得到了实验验证,具体脉冲波形见图S1.在实验前,对包含Cr、胆碱(Cholin,Cho)以及GSH的五自旋体系算符随脉冲施加演化轨迹模拟如图S2所示,模拟表明OC脉冲可以高效的完成对相应算符的转化,具有进一步开展活体实验的理论基础. 使用西门子MAGNETOM 3T-Prisma成像仪平台进行测试,实验共招募了5位健康志愿者,年龄为22~27岁,包含4位男性和1位女性.实验通过了华东师范大学伦理委员会的批准(批准文号HR 167-2024).实验结果如图5所示,(a)为所选体素位置结构相示意图,(b)为使用PRESS序列采集的常规1H-MRS谱.此处仅展示了化学位移区间2.5 ppm~3.5 ppm内的谱图,其中2.95 ppm的GSH信号完全被包埋于Cr的尖峰中.2.96 ppm附近还包含有GABA的信号.该区域三种分子的信号重叠严重.(c)为施加了GSH-SFOC滤波脉冲之后的1H-MRS谱,位于2.95 ppm的信号即为GSH中Cys-CH2信号,Cr和GABA的信号几乎完全被抑制.
图5
图5
基于Siemens 3T-Prisma成像仪平台的人脑活体GSH分子靶向1H-MRS图. (a)所选体素位置结构相示意图,其中白色方框即为所选体素范围;(b)运用PRESS序列采集的1H-MRS谱图,截取2.5 ppm~3.5 ppm范围的谱图;(c)应用GSH-SFOC方法采集的5位不同被试者的1H-MRS谱图,截取2.5 ppm~3.5 ppm范围的谱图,谱图被放大5倍. 其中用方框标记的范围为GSH中Cys-CH2的信号. 所有1H-MRS谱图均充零至32 k个数据点,运用大小为3.8的高斯窗函数进行了处理
Fig. 5
Results of in vivo GSH molecular targeted 1H-MRS in human brain collected on the Siemens 3T-Prisma imaging system. (a) The schematic diagram of the selected voxel position with the white box indicating the selected voxel range. (b) The 1H-MRS spectrum acquired using the PRESS sequence, with a schematic representation of the 2.5~3.5 ppm range. (c) The 1H-MRS spectra collected using the SFOC method, with the spectra magnified by 5 times and the 2.5~3.5 ppm range highlighted in the box indicating the GSH Cys-CH2 signal. All 1H-MRS spectra were zero-filled to 32 k data points and processed using a Gaussian window function with a size of 3.8
表1 活体GSH浓度定量分析
Table 1
| 编号 | 体素大小/cm3 | SFOC目标峰面积 | 浓度/(mmol/L) |
|---|---|---|---|
| 1(水模) | 2×3×2 | 695.46 | 10 |
| 2 | 2×3×2 | 81.79 | 1.16 |
| 3 | 2×3×2 | 86.49 | 1.23 |
| 4 | 2×3×2 | 56.68 | 0.81 |
| 5 | 2×3×2 | 96.71 | 1.38 |
| 6 | 2×3×2 | 86.51 | 1.23 |
注:以人脑活体GSH靶向1H-MRS数据与已知浓度水模GSH-SFOC-MRS滤波后的目标信号峰面积的积分为计算参数,获取了5位被试者顶叶体素中的GSH浓度. 计算所使用的公式为
在活体中的实验结果证明GSH-SFOC滤波方法能够用于GSH分子的选择性检测及定量.该方法能较好地压制与GSH重叠的Cr和GABA的信号,有助于GSH分子的准确定量.
在本方法提出之前,Choi等人提出的dual-DQC脉冲也能通过一次扫描获得GSH分子靶向谱图,该方法通过半选择性脉冲制备双量子态实现信号选择[16].本文模拟了GSH-SFOC-MRS方法的理论效率并与dual-DQC对比,模拟结果见图S4. 针对3 T上GSH分子自旋的特点,dual-DQC方法的理论滤波效率为49%.为了得到相同的信号强度,GSH-SFOC-MRS脉冲所需的采样时间远短于dual-DQC脉冲的时间.
4 总结
本文提出了一种针对GSH分子的滤波方法,该方法通过施加优化脉冲将GSH分子中的两个氢原子核制备为核自旋单重态,利用单重态的性质实现分子的靶向信号选择.脉冲序列分别在水模和活体人脑中进行了实验验证,实验结果表明本文的滤波方法能有效地对GSH分子信号进行选择性检测,同时还能有效地验证与之重合的Cr和GABA的信号.该脉冲序列有望进一步应用于人脑不同区域GSH信号的检测,为研究与GSH相关的疾病或者生理过程提供直接的实验依据.
利益冲突
无
附件材料
附件材料(可在《波谱学杂志》官网
图S1 OC脉冲波形图
图S2 OC脉冲算符演化轨迹示意图
图S3 浓度梯度水模实验拟合
图S4 SFOC和dual-DQC效率模拟对比图
参考文献
Glutathione pathways in the brain
[J].
DOI:10.1515/BC.2003.059
PMID:12751781
[本文引用: 1]

The antioxidant glutathione (GSH) is essential for the cellular detoxification of reactive oxygen species in brain cells. A compromised GSH system in the brain has been connected with the oxidative stress occuring in neurological diseases. Recent data demonstrate that besides intracellular functions GSH has also important extracellular functions in brain. In this respect astrocytes appear to play a key role in the GSH metabolism of the brain, since astroglial GSH export is essential for providing GSH precursors to neurons. Of the different brain cell types studied in vitro only astrocytes release substantial amounts of GSH. In addition, during oxidative stress astrocytes efficiently export glutathione disulfide (GSSG). The multidrug resistance protein 1 participates in both the export of GSH and GSSG from astrocytes. This review focuses on recent results on the export of GSH and GSSG from brain cells as well as on the functions of extracellular GSH in the brain. In addition, implications of disturbed GSH pathways in brain for neurodegenerative diseases will be discussed.
Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression
[J].
DOI:10.5607/en.2014.23.1.93
PMID:24737944
[本文引用: 1]

Glutathione (GSH) protects cells against oxidative stress by playing an antioxidant role. Protecting brain endothelial cells under oxidative stress is key to treating cerebrovascular diseases and neurodegenerative diseases including Alzheimer's disease and Huntington's disease. In present study, we investigated the protective effect of GSH on brain endothelial cells against hydrogen peroxide (H2O2). We showed that GSH attenuates H2O2-induced production of nitric oxide (NO), reactive oxygen species (ROS), and 8-Oxo-2'-deoxyguanosine (8-OHdG), an oxidized form of deoxiguanosine. GSH also prevents H2O2-induced reduction of tight junction proteins. Finally, GSH increases the level of nuclear factor erythroid 2-related factor 2 (Nrf2) and activates Nrf2-mediated signaling pathways. Thus, GSH is a promising target to protect brain endothelial cells in conditions of brain injury and disease.
Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy
[J].
Role of glutathione in cancer: From mechanisms to therapies
[J].
Changes in glutathione content in liver diseases: An update
[J].
Glutathione in liver diseases and hepatotoxicity
[J].
Gender differences in glutathione metabolism in Alzheimer's disease
[J].The mechanism underlying Alzheimer's disease (AD), an age-related neurodegenerative disease, is still an area of significant controversy. Oxidative damage of macromolecules has been suggested to play an important role in the development of AD; however, the underlying mechanism is still unclear. In this study, we showed that the concentration of glutathione (GSH), the most abundant intracellular free thiol and an important antioxidant, was decreased in red blood cells from male AD patients compared with age- and gender-matched controls. However, there was no difference in blood GSH concentration between the female patients and female controls. The decrease in GSH content in red blood cells from male AD patients was associated with reduced activities of glutamate cysteine ligase and glutathione synthase, the two enzymes involved in de novo GSH synthesis, with no change in the amount of oxidized glutathione or the activity of glutathione reductase, suggesting that a decreased de novo GSH synthetic capacity is responsible for the decline in GSH content in AD. These results showed for the first time that GSH metabolism was regulated differently in male and female AD patients.Copyright 2005 Wiley-Liss, Inc.
Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo
[J].
DOI:10.1046/j.1460-9568.2000.00229.x
PMID:11029642
[本文引用: 1]

Schizophrenia is a major psychiatric disease, which affects the centre of the personality, with severe problems of perception, cognition as well as affective and social behaviour. In cerebrospinal fluid of drug-free schizophrenic patients, a significant decrease in the level of total glutathione (GSH) by 27% (P<0.05) was observed as compared to controls, in keeping with the reported reduced level of its metabolite gamma-glutamylglutamine. With a new non-invasive proton magnetic resonance spectroscopy methodology, GSH level in medial prefrontal cortex of schizophrenic patients was found to be 52% (P = 0.0012) lower than in controls. GSH plays a fundamental role in protecting cells from damage by reactive oxygen species generated among others by the metabolism of dopamine. A deficit in GSH would lead to degenerative processes in the surrounding of dopaminergic terminals resulting in loss of connectivity. GSH also potentiates the N-methyl-D-aspartate (NMDA) receptor response to glutamate, an effect presumably reduced by a GSH deficit, leading to a situation similar to the application of phencyclidine (PCP). Thus, a GSH hypothesis might integrate many established biological aspects of schizophrenia.
Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease
[J].
Magnetic resonance spectroscopy in AD
[J].Proton MR spectroscopy (MRS) studies have found both decreased N-acetylaspartate (NAA) and increased myo-inositol in the occipital, temporal, parietal, and frontal regions of patients with AD, even at the early stages of the disease. This diffuse NAA decline is independent of regional atrophy and probably reflects a decrease in neurocellular viability. Reports of such metabolite changes are now emerging in the mild cognitive impairment prodrome and in investigation of the medial temporal lobe. In vivo quantitation of neural choline in AD has been inconclusive because of poor test-retest repeatability. Less robust evidence using phosphorous MRS has shown significant phosphocreatine decline and increments in the cell membrane phosphomonoesters in the early, and possibly asymptomatic, stages of the disease. These phosphorous metabolite disturbances normalize with disease progression. Phosphodiester concentration has been found to correlate strongly with AD plaque counts. MRS of AD has therefore introduced new pathophysiologic speculations. Studies of automated MRS for AD diagnosis have reported high sensitivity and moderate specificity, but are yet to test prospective samples and should be extended to include at least two MRS regions of interest. MRS has promise for predicting cognitive status and monitoring pharmacologic efficacy, and can assess cortical and subcortical neurochemical change.
GABA-induced motor improvement following acute cerebral infarction
[J].γ-Aminobutyric acid (GABA) plays a key role in motor learning. In the aftermath of stroke, we monitored GABA+ content of primary motor cortex by magnetic resonance spectroscopy (MRS), assessing its relation to functional motor recovery following a standardized 4-week program of rehabilitation. The cohort included 20 patients, each experiencing stroke within 2 weeks of symptom onset. Twenty age-matched healthy subjects were also recruited as controls. GABA+ levels were determined at baseline and following rehabilitation, performed only once in sex- and age-matched control subjects. Motor functions were then measured via Fugl-Meyer Assessment (FMA). Processing of MRS data was driven by open-source Gannet software. Because GABA, macromolecules, and homocarnosine jointly contribute to MEscher-Garwood Point RESolved Spectroscopy (MEGA-PRESS) signals, the designation GABA+ (rather than GABA) was applied. Baseline GABA+/creatine (Cr) ratios proved significantly lower in patients with strokes than in control subjects (<0.05). Following the 4-week rehabilitative regimen, significant improvement in FMA indices was evident across the test group. FMA scores and GABA+/Cr ratios correlated significantly at baseline, the GABA+/Cr ratio displaying a significant association with motor function (=0.025). In the setting of acute stroke, GABA+/Cr ratios of primary motor cortex fell significantly below levels found in healthy subjects. The observed association between GABA+/Cr ratio and motor recovery underscores the utility of MRS-measured GABA as a key motor recuperative biomarker.AJTR Copyright © 2020.
Enhancement of spectral editing efficacy of multiple quantum filters in in vivo proton magnetic resonance spectroscopy
[J].
DOI:10.1016/j.jmr.2012.07.017
PMID:22975239
[本文引用: 1]

The performance of multiple quantum filters (MQFs) can be disappointing when the background signal also arises from coupled spins. Moreover, at 3.0 T and even higher fields the majority of the spin systems of key brain metabolites fall into the strong-coupling regime. In this manuscript we address comprehensively, the importance of the phase of the multiple quantum coherence-generating pulse (MQ-pulse) in the design of MQFs, using both product operator and numerical analysis, in both zero and double quantum filter designs. The theoretical analyses were experimentally validated with the examples of myo-inositol editing and the separation of glutamate from glutamine. The results demonstrate that the phase of the MQ-pulse per se provides an additional spectral discrimination mechanism based on the degree of coupling beyond the conventional level-of-coherence approach of MQFs. To obtain the best spectral discrimination of strongly-coupled spin systems, therefore, the phase of the MQ-pulse must be included in the portfolio of the sequence parameters to be optimized.Copyright © 2012 Elsevier Inc. All rights reserved.
Brain glutathione levels-A novel biomarker for mild cognitive impairment and Alzheimer’s disease
[J].
Single-session reproducibility of MR spectroscopy measures of glutathione in the mesial temporal lobe with MEGA-PRESS
[J].
Doubly selective multiple quantum chemical shift imaging and T1 relaxation time measurement of glutathione (GSH) in the human brain in vivo
[J].
Measurement of glutathione in human brain at 3T using an improved double quantum filter in vivo
[J].
Preparation of nuclear spin singlet states using spin-lock induced crossing
[J].
Singlet NMR methodology in two-spin-1/2 systems
[J].
Theory of long-lived nuclear spin states in solution nuclear magnetic resonance. II. Singlet spin locking
[J].
Optimal control of coupled spin dynamics: design of NMR pulse sequences by gradient ascent algorithms
[J].In this paper, we introduce optimal control algorithm for the design of pulse sequences in NMR spectroscopy. This methodology is used for designing pulse sequences that maximize the coherence transfer between coupled spins in a given specified time, minimize the relaxation effects in a given coherence transfer step or minimize the time required to produce a given unitary propagator, as desired. The application of these pulse engineering methods to design pulse sequences that are robust to experimentally important parameter variations, such as chemical shift dispersion or radiofrequency (rf) variations due to imperfections such as rf inhomogeneity is also explained.
Distinguishing glutamate and glutamine in in vivo 1H MRS based on nuclear spin singlet order filtering
[J].
Selectively probing the magnetic resonance signals of γ-aminobutyric acid in human brains in vivo
[J].
Preparation efficiency of singlet states in multi-spin systems with different coupling configurations
[J].
不同耦合构型多自旋体系单重态制备效率研究
[J].
DOI:10.11938/cjmr20233063
[本文引用: 1]

核自旋单重态是一种特殊的量子态,其存在寿命能远长于纵向弛豫时间(T<sub>1</sub>),能够被用于研究分子之间的慢扩散和慢运动等动力学过程.单重态的制备是其能成功应用的关键.目前文献中报道了多种制备单重态的方法,这些方法主要适用于孤立的两自旋体系,当单重态所涉及的核自旋受到其它自旋的耦合作用时,单重态的制备效率往往会降低.本文以三自旋体系为例,研究了不同耦合构型下单重态的制备效率受非单重态自旋耦合的影响,模拟结果表明当单重态自旋由弱耦合逐渐变为强耦合时,单重态的制备效率会在单重态自旋与非单重态自旋的耦合呈现对称性时保持一定的稳定性,此特性能够为复杂体系中选择合适的自旋制备单重态提供参考.我们利用N-乙酰-L-天门冬氨酸(NAA)分子的三个质子组成的自旋体系实验验证了以上结论,通过调节NAA分子的酸碱度,所选的三自旋体系能从弱耦合向强耦合转变,实验结果表明:当三自旋中单重态自旋处于强耦合时,单重态的制备效率明显高于弱耦合时的效率.
Preparing nuclear spin singlet state in a three-spin system and its application in 2D spectrum
[J].
三自旋体系核自旋单重态的制备与单重态二维谱的实现
[J].
DOI:10.11938/cjmr20212910
[本文引用: 1]

核自旋单重态是一种特殊的自旋状态,其寿命远长于相应自旋的横向和纵向弛豫时间,能够被用于研究分子的慢扩散、慢运动、特征信号选择等过程.目前单重态的研究主要集中于孤立的两自旋体系.而本文以N-乙酰基天冬氨酸(NAA)分子中由亚甲基和次甲基的三个氢原子核构成的三自旋体系为研究对象,将亚甲基中的两个氢核制备成单重态.利用优化控制和数值计算方法,分别设计了包含和不包含次甲基氢核耦合的单重态制备脉冲,结果发现,不考虑次甲基氢耦合设计的优化脉冲,其在实际三自旋体系中的单重态制备效率会显著下降.另外,我们以单重态为起点,实现了针对次甲基和亚甲基的信号选择COSY谱和NOESY谱,结果表明基于单重态的二维谱能够有效避免谱峰重叠现象,提高谱图分辨率,并有助于提高分子结构解析的准确性.
Spatial localization in NMR spectroscopy in vivo
[J].
Long-lived nuclear spin states in high-field solution NMR
[J].Nuclear spin order may be stored in a liquid for a much longer time than the longitudinal relaxation time T1, by using rf fields to isolate states of different symmetry. The method is demonstrated on a sample containing AX spin systems.
Singlet nuclear magnetic resonance of nearly-equivalent spins
[J].
DOI:10.1039/c0cp02293d
PMID:21318206
[本文引用: 1]

Nuclear singlet states may display lifetimes that are an order of magnitude greater than conventional relaxation times. Existing methods for accessing these long-lived states require a resolved chemical shift difference between the nuclei involved. Here, we demonstrate a new method for accessing singlet states that works even when the nuclei are almost magnetically equivalent, such that the chemical shift difference is unresolved. The method involves trains of 180° pulses that are synchronized with the spin-spin coupling between the nuclei. We demonstrate experiments on the terminal glycine resonances of the tripeptide alanylglycylglycine (AGG) in aqueous solution, showing that the nuclear singlet order of this system is long-lived even when no resonant locking field is applied. Variation of the pulse sequence parameters allows the estimation of small chemical shift differences that are normally obscured by larger J-couplings.
Detection of glutathione in the human brain in vivo by means of double quantum coherence filtering
[J].The feasibility of selective in vivo detection of glutathione (L-gamma-glutamyl-L-cysteinyl-glycine, GSH) in the human brain by means of (1)H magnetic resonance spectroscopy (MRS) at 1.5 T is demonstrated. A double quantum coherence (DQC) filtering sequence was used in combination with PRESS volume selection. The strongly coupled cysteinyl CH(2) compound of GSH was found to be the most suitable target for spectral editing. Analytical calculations employing a product operator description of the cysteinyl ABX three-spin system were made in order to optimize the inherent yield of the sequence. A pulse phase calibration procedure, which precedes the spectrum acquisition, secures maximal signal yield independently of the spatial localization of the volume of interest and thus comparability between individual examinations. In vitro tests show that the DQC filtering method provides good discrimination between the GSH signal at 2.9 ppm and the interfering resonances of creatine, gamma-aminobutyric acid (GABA) and aspartate. In measurements in the frontal lobe of 12 healthy volunteers a mean ratio of GSH signal to tissue water signal of 5.7 +/- 2.3 x 10(-5) was found, corresponding to a mean GSH tissue concentration of 2-5 mmol/L. The proposed technique allows for the detection of a biologically highly relevant metabolite at moderate field strength. Magn Reson Med 42:283-289, 1999.Copyright 1999 Wiley-Liss, Inc.
In vivo brain GSH: MRS methods and clinical applications
[J].