引言
然而,由医生在TOF-MRA影像上手动标注动脉区域需要大量的时间和精力,因此开发一种脑动脉树区域自动分割方法非常必要.
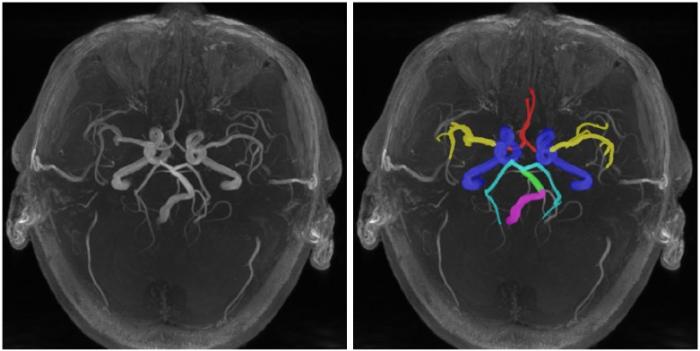
与已有的大量的动脉树提取研究[6⇓⇓-9]相比较,由于动脉树的区域分割需要更进一步,因此目前的研究相对较少.如图1中3D TOF-MRA的轴向最大密度投影(maximal intensity projection,MIP)所示,颅内动脉树通常会按照Willis环[10]的解剖学结构将分支分为颈内动脉(internal carotid arteries,ICA,蓝色)、基底动脉(basilar artery,BA,绿色)、椎动脉(vertebral artery,VA,紫色)、大脑中动脉(middle cerebral artery,MCA,黄色)、大脑前动脉(anterior cerebral artery,ACA,红色)和大脑后动脉(posterior cerebral artery,PCA,青色)这6个主要区域.Akihiro等[11]统计了7个MRA影像并绘制了动脉区域范围模板,他们基于模板完成动脉树自动分区,但该方法忽略了VA区域,在ACA区域的准确度不足30%.之后,Nowinski等[12]开发了一种半自动颅内动脉重建工具,能够为健康受试者建立具有完整标记的动脉分布图,然而该工具需要大量的人工参与.最近,Li等[13,14]基于前人的工作,开发了一种新型动脉特征提取工具iCafe,iCafe通过深度学习模型自动提取完整颅内动脉树,之后使用概率模型在模板库中匹配分支所属区域,但iCafe要求使用者具有随时纠正匹配错误和细节错误的能力.综上,现有的动脉树分区方法存在两点主要缺陷:(1)需要具有一定专业水平的人工参与;(2)易发生分区错误,尤其是在前、后动脉区域易发生混淆.
图1
图1
颅内动脉的6个主要区域:颈内动脉(ICA,蓝色)、基底动脉(BA,绿色)、椎动脉(VA,紫色)、大脑中动脉(MCA,黄色)、大脑前动脉(ACA,红色)和大脑后动脉(PCA,青色)
Fig. 1
6 regions of intracranial arteries: internal carotid arteries (ICA, blue), basilar artery (BA, green), vertebral artery (VA, purple), middle cerebral artery (MCA, yellow), anterior cerebral artery (ACA, red) and posterior cerebral artery (PCA, cyan)
神经网络已经在许多医学影像分割任务中被验证有效[15,16],但在动脉树分区任务中存在两个主要问题:(1)网络的下采样操作会造成细小动脉的特征丢失;(2)动脉区域划分是一种位置敏感的任务,而卷积层只能提取感受野内的局部特征.针对以上问题,本文采用了一种基于双分支网络[17,18]改进的双分支连通网络(dual branch connected network,DBCNet).该网络使用了分支解耦模块(bifurcation attention,BiA)和基于Swin Transformer[19]的Swin-Crisscross(SC)模块以提升网络分割性能,整套方法实现了从原始数据直接得到动脉区域分割结果的端到端输出.
1 实验部分
1.1 实验数据
本研究的实验数据来源于上海复旦大学附属华山医院,均从临床常规工作中收集.本回顾性研究已获得合作医院机构伦理委员会的批准.所有脑部动脉影像收集自2016年1月至2018年2月,采集设备为GE MEDICAL SYSTEMS DISCOVERY MR750,核磁共振场强3.0 T,头部线圈通道32,使用3D TOF-MRA序列.表1详细展示了本研究数据在采集时使用的参数.剔除了成像质量不佳和有伪影的影像后,最终的111例数据中包含健康人影像54例,颅内动脉瘤患者影像57例.分区的标注由一位高年资医师使用标注软件ITK-SNAP[20]指定各区域血管段的起始点坐标,由低年资医师完成血管段的标注.由于远端动脉分支多且复杂,成像不清晰,标注难度大,且发病率远低于近端动脉[21],因此本研究中不考虑远端动脉的分区.本研究随机选取5例健康人数据和5例动脉瘤阳性数据影像作为测试集,剩余数据中81例作为训练集,20例作为验证集.
表1 数据采集参数表
Table 1
| 血管疾病 | 重复时间 | 回波时间 | 视野百分比相位 | 采集矩阵 | 层内分辨率 | 图像层数 | 反转角 | 层厚 |
|---|---|---|---|---|---|---|---|---|
| 健康人 | 3.4 ms | 25 ms | 88% | 320×192 | 0.43 mm×0.43 mm | 128 | 20° | 1.4 mm |
| 动脉瘤患者 | 5.7 ms | 25 ms | 88% | 320×256 | 0.21 mm×0.21 mm | 240 | 20° | 1.2/1.4 mm |
注:动脉瘤患者的影像为初次TOF-MRA检查后由医师筛查为患者后的二次扫描结果,因此参数与健康人的TOF-MRA影像有所差异.本研究所使用的111例影像中健康人与动脉瘤患者无交集.
1.2 数据处理
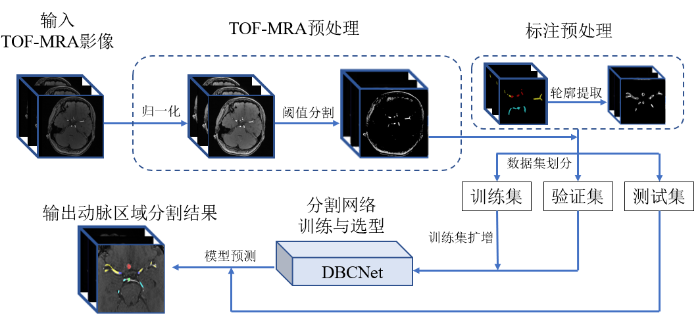
本文提出方法的主要实验流程如图2所示,首先对TOF-MRA进行预处理,并对标注执行轮廓提取,处理后的数据和对应标注划分为训练集、验证集和测试集,使用扩增后的训练集训练DBCNet并在验证集上评估模型分割结果,将得到的最佳网络模型在测试集完成性能评估.
图2
1.2.1 数据预处理
数据预处理分为TOF-MRA预处理、标注预处理和数据扩增.
(1)TOF-MRA预处理.首先进行归一化,将3D TOF-MRA影像的灰度范围映射到0~1 024并重采样至各向同性.然后基于直方图统计,利用阈值分割去除大部分灰度较低的脑组织.
(2)标注预处理.DBCNet的训练过程需要在定位分支和分割分支同时输入真实值.将标注图像作为分割分支的真实值,并将标注映射为二值图像后,使用Canny算子[22]计算其轮廓作为定位分支的真实值.
(3)数据扩增.训练集和验证集按照约4:1的比例划分后,对训练集使用翻转,添加噪声和直方图均衡化3种方法完成8倍扩增.
1.3 DBCNet
图3
图3
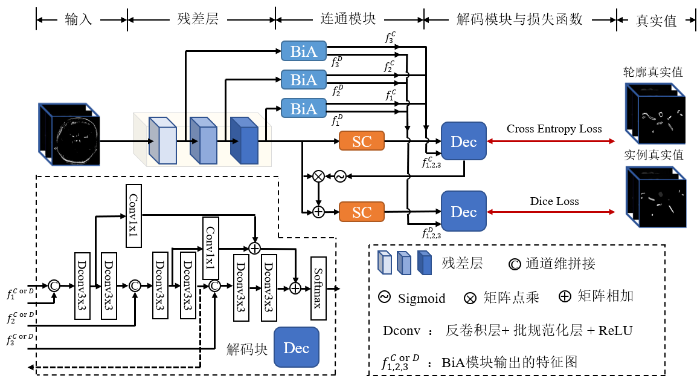
DBCNet网络架构图.其中,Dec为网络的解码块,BiA和SC是本研究提出的分支解耦模块和深层特征提取模块.得到BiA模块的最终输出特征图$f_{i}^{\text{C}}$和$f_{i}^{\text{D}}$,其中C和D分别表示定位分支和分割分支,i取1、2、3代表不同的BiA模块
Fig. 3
The architecture diagram of DBCNet network. Where Dec is the decoding block of the network, BiA and SC are the branch decoupling module and deep feature extraction module proposed in this study. The final output feature maps $f_{i}^{\text{C}}$ and $f_{i}^{\text{D}}$ of the BiA module are obtained, where C and D represent the localization branch and the segmentation branch, respectively, and i takes 1, 2 and 3 to represent different BiA modules
编码侧残差层之后分为定位分支和分割分支,两条分支在训练中分别由轮廓真实值和实例真实值监督学习.每条分支均由SC模块和解码块组成,其中解码块与编码路径类似,分为三个不同尺度.每个尺度由两个反卷积块组成,反卷积块由反卷积层、批规范化层和ReLU激活层组成,第一个反卷积块的输出除进入下一层外,还会通过1×1卷积层调整通道数后与下一个尺度的特征图相加,起到深监督作用.通过BiA模块的特征图会与对应尺度的反卷积块输入进行拼接.定位分支中第二个反卷积块的输出通过两个残差层完成下采样,之后通过Sigmoid函数得到权重图与进入分支时的残差层特征图点乘后相加,作为分割分支的输入.解码块的最后一层是SoftMax层,可以得到每个体素所属区域的概率.
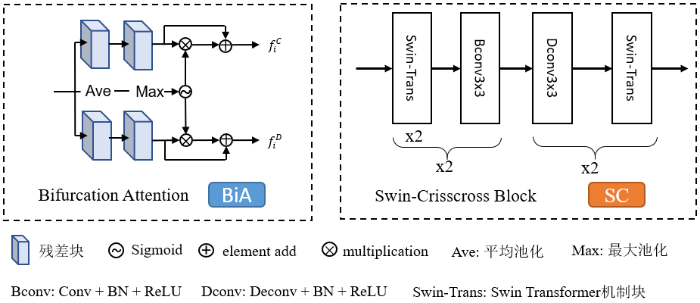
1.3.1 BiA模块
图4
1.3.2 SC模块
1.3.3 损失函数和两步训练策略
DBCNet使用的损失函数为交叉熵(Cross Entropy)和Dice损失函数的权重和,损失函数如(1)~(3)式所示.
(1)式中,i代表定位分支中轮廓前景值1和背景值0,$P(i)$和$Q(i)$分别代表预测为前景或背景的概率值和真实值.(2)式中,N为体素总个数,$y(j)$和$\hat{y}(j)$分别表示体素j的标签值和预测值.(3)式中,α和β代表赋予不同交叉熵和Dice损失函数的权重值.
DBCNet的定位分支存在真实值前景与背景体素不均衡的问题,而交叉熵损失函数在进行前景、背景体素数量严重不均衡的二分类任务时会使模型具有偏向性[25],在定位分支使用交叉熵损失函数仅用于检测和定位动脉区域.而Dice损失函数在训练时更关注对前景区域的挖掘,可以缓解样本中前景与背景不平衡带来的消极影响[25],因此分割分支的Dice损失函数权重更高时,模型便倾向于实现更精细的分割.针对两种损失函数的特点,本研究采用了一种两步训练策略进行网络模型的训练.首先,设置$\alpha =0.9$和$\beta =0.1$进行训练,为交叉熵损失赋予较高权重,促使网络更关注定位分支.之后,在损失函数L连续20次不下降后,交换α和β的值,模型便可以在完成动脉定位之后再去关注血管区域的分割.
1.4 模型评估
本研究使用(2)式中的Dice系数和95%豪斯多夫距离(HD95)来评估模型,HD95可以表示预测值和真实值之间的表面距离,其公式为:
其中,N代表预测值的通道数;d表示计算两个集合间的距离;$\underset{95%}{\mathop{\max }}\,$表示取集合间距离的95百分位数;$X(i)$和$Y(i)$代表第i通道的预测结果和真实值.
1.5 训练环境及参数
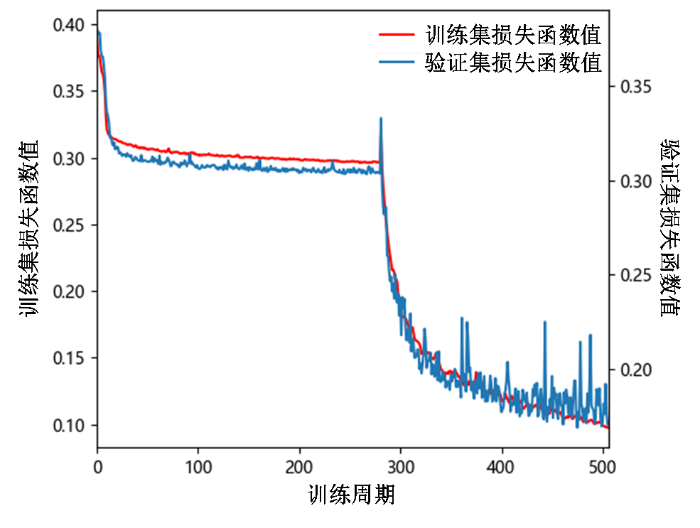
本研究的所有实验均基于i7-10700K CPU和一块NVIDIA GeForce RTX 3080 Ti GPU进行;系统和主要软件环境为Windows 10、Python 3.7.11和PyTorch 1.9.0.相关参数设置为:网络模型输入图像需重采样至64×128×128;批处理大小为1;初始学习率为0.002,训练每迭代20个周期,学习率衰减为上一周期的0.9;训练采用早停法,早停在损失函数的权重交换完成后开启,连续30次模型在验证集上计算的损失值不下降,则停止训练,并选择训练过程中在验证集上表现最优的模型作为测试用模型.
2 结果与讨论
2.1 基于DBCNet的颅内动脉树区域自动分割
图5
图6
图6
DBCNet颅内动脉树区域分割预测结果的三维重建展示图. A行为1例健康人的动脉树三维重建(对TOF-MRA进行阈值分割得到)、标注真实值和分割结果展示;B行为1例颅内动脉瘤患者的动脉树三维重建、标注真实值和分割结果展示,患者动脉瘤在大脑前动脉(ACA)区域.真实值和模型分割结果视觉效果不同,是因为标注真实值为人工使用实心小球绘制,而模型分割结果是由体素级分割后上采样回原图像大小得到的. 颈内动脉:ICA,蓝色;基底动脉:BA,绿色;椎动脉:VA,紫色;大脑中动脉:MCA,黄色;大脑前动脉:ACA,红色大脑后动脉:PCA,青色
Fig. 6
3D reconstruction for DBCNet intracranial arterial tree region segmentation prediction results. Row A shows the arterial tree 3D reconstruction (threshold segmentation result of TOF-MRA), labeled real values and segmentation results of a healthy person; row B shows the arterial tree 3D reconstruction, labeled real values and segmentation results of a patient with intracranial aneurysm, the patient’s aneurysm is in the anterior cerebral artery (ACA) region. The visual effect of the real values and model segmentation results is different because the labeled real values are drawn manually using solid spheres, while the model segmentation results are obtained by up-sampling back to the original image size after voxel-level segmentation. Internal carotid arteries (ICA, blue), basilar artery (BA, green), vertebral artery (VA, purple), middle cerebral artery (MCA, yellow), anterior (ACA, red) and posterior cerebral artery (PCA, cyan)
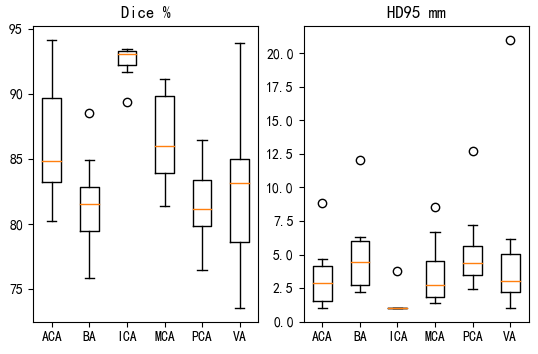
表2展示了利用训练好的DBCNet模型对10例测试数据进行分割的性能评估结果.其中,ACA区域的平均Dice系数和平均HD95分别为86.32%和3.30 mm,BA区域分别为81.56%和4.94 mm,ICA区域分别为92.52%和1.27 mm,MCA区域分别为86.53%和3.64 mm,PCA区域分别为81.66%和5.16 mm,VA区域分别为82.92%和5.05 mm,完整动脉树分别为74.72%和3.89 mm.图7绘制了10例测试数据各区域分割结果的Dice系数和HD95箱形图,可见ICA区域的分割结果最佳,10例数据间的波动最小,而VA区域的分割结果波动大且存在偏差较大的异常值.
表2 10例测试数据中颅内动脉树各区域的分割性能评估
Table 2
| ACA | BA | ICA | MCA | PCA | VA | 平均 | |
|---|---|---|---|---|---|---|---|
| Dice/% | 86.32±4.59 | 81.56±3.54 | 92.52±1.25 | 86.53±3.44 | 81.66±3.15 | 82.92±6.28 | 74.72±3.36 |
| HD95/mm | 3.30±2.32 | 4.94±2.97 | 1.27±0.87 | 3.64±2.41 | 5.16±3.02 | 5.05±5.85 | 3.89±1.30 |
注:表格中数据为均值±方差.
图7
图7
10例测试集数据各区域分割结果的Dice系数和HD95箱形图
Fig. 7
Box plots of Dice coefficients and HD95 for each region segmentation in the testing data set
2.2 不同网络架构的对比
表3 利用DBCNet与常见深度学习网络对测试集数据进行分割的性能评估
Table 3
| ACA | BA | ICA | MCA | PCA | VA | 平均 | ||
|---|---|---|---|---|---|---|---|---|
| nnUNet | Dice/% | 52.18±2.29 | 0 | 80.03±5.93 | 57.00±2.15 | 45.00±15.81 | 0 | 26.78±3.91 |
| HD95/mm | 31.74±8.50 | 30 | 8.29±11.01 | 21.91±14.10 | 27.15±4.08 | 30 | 24.84±3.94 | |
| Modified UNet | Dice/% | 77.89±5.14 | 81.01±7.22 | 89.49±2.67 | 76.26±2.94 | 0 | 0 | 49.90±3.59 |
| HD95/mm | 6.48±4.48 | 8.49±14.01 | 4.95±10.67 | 7.99±3.32 | 30 | 30 | 14.65±2.69 | |
| VNet | Dice/% | 58.70±22.74 | 73.56±7.79 | 89.46±3.81 | 70.86±4.16 | 72.97±5.66 | 70.04±14.56 | 54.56±8.59 |
| HD95/mm | 13.56±8.43 | 12.59±14.59 | 5.16±11.03 | 15.76±4.84 | 22.81±6.57 | 14.33±6.69 | 14.04±8.69 | |
| DBCNet | Dice/% | 86.32±4.59 | 81.56±3.54 | 92.52±1.25 | 86.53±3.44 | 81.66±3.15 | 82.92±6.28 | 74.72±3.36 |
| HD95/mm | 3.30±2.32 | 4.94±2.97 | 1.27±0.87 | 3.64±2.41 | 5.16±3.02 | 5.05±5.85 | 3.89±1.30 |
图8
图8
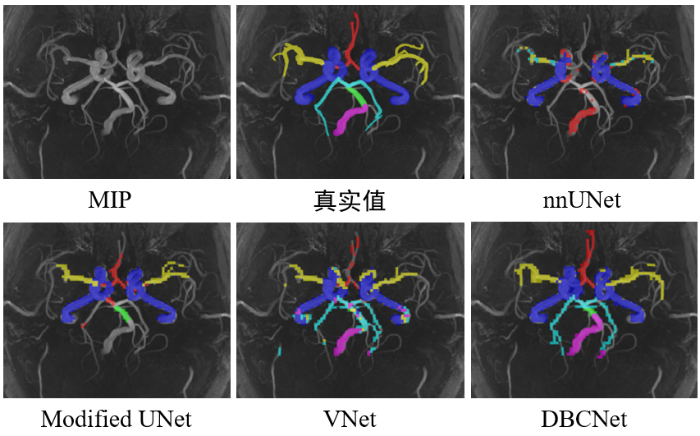
在MIP上不同深度学习网络对健康人的3D TOF-MRA影像的动脉树分割结果
Fig. 8
Arterial tree segmentations using different deep learning networks for 3D TOF-MRA images of a healthy people subject on MIP
图9
图9
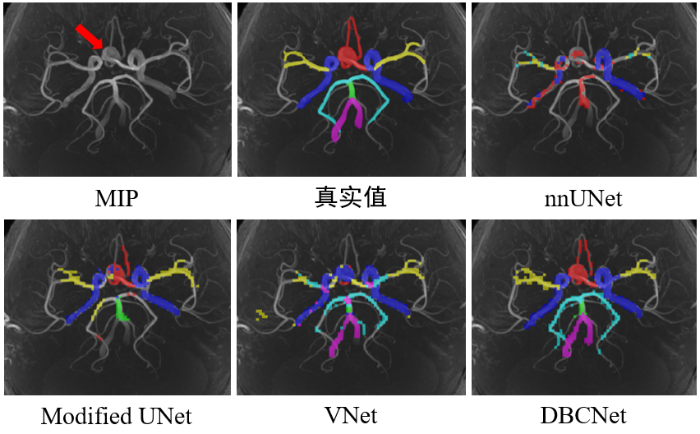
在MIP上不同深度学习网络对颅内动脉瘤患者的3D TOF-MRA影像的动脉树分割结果
Fig. 9
Arterial tree segmentations using different deep learning networks for 3D TOF-MRA images of intracranial aneurysm patients on MIP
2.3 消融实验
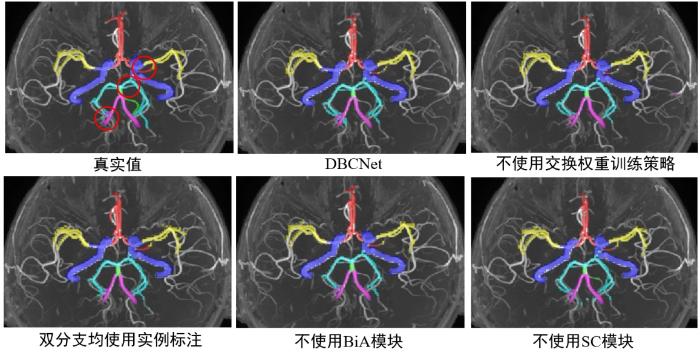
为了验证本文提出的改进方法和运用策略的有效性,在验证集进行了消融实验.如表4所示,完整的DBCNet模型预测结果的Dice系数平均值为87.36%,HD95平均值为1.47 mm.不使用交换权重的两步训练策略,固定损失函数中Dice的权重为0.9,交叉熵的权重为0.1,预测结果的Dice系数平均值下降5.20%,HD95增加0.37 mm,这是因为使用两步训练策略可以在动脉区域分割前先对模型进行针对动脉结构的预训练,提升最终分割效果.去除BiA和SC模块后,模型在Dice系数上均有不同程度的下降,HD95有所增加,说明两种模块均可以提升模型的特征提取能力.考虑到采用双分支网络的目的是为了向网络中注入额外的动脉特征,因此研究将双分支的真实值均采用分割分支的实例标注,得到的Dice系数平均值下降3.03%,HD95增加0.14 mm,这证明使用外轮廓作为真实值起到了增加额外动脉特征的作用.图10中展示了使用不同消融策略进行消融实验的结果.
表4 DBCNet分割脑动脉区域在验证集中的消融实验
Table 4
| 模型 | 消融策略 | Dice/% | HD95/mm |
|---|---|---|---|
| DBCNet | 无 | 87.36 | 1.47 |
| 不使用交换权重训练策略 | 82.16 (↓5.20) | 1.84 (↑0.37) | |
| 双分支均使用实例标注 | 84.33 (↓3.03) | 1.61 (↑0.14) | |
| 不使用BiA模块 | 84.53 (↓2.83) | 1.90 (↑0.43) | |
| 不使用SC模块 | 84.62 (↓2.74) | 2.00 (↑0.53) |
注:↓和↑分别表示与完整DBCNet相比,指标值下降或上升,括号中的数字表示下降或上升的程度.
图10
图10
在MIP上展示消融实验中3D TOF-MRA影像的动脉树分割结果,真实值的红圈位置在不同策略分割结果中有相对明显的差异
Fig. 10
Arterial tree segmentations of ablation experimental for 3D TOF-MRA images of intracranial aneurysm patients on MIP. The red circles in the ground truth image indicate the apparent differences in segmentation results generated by different strategies
2.4 讨论
本文提出了一种用于在3D TOF-MRA影像中分割脑动脉树区域的方法,在搭建深度学习网络DBCNet时,设计了将双分支特征解耦的BiA模块,缓解了双分支网络使用长连接时的特征干涉问题,又设计了SC模块使网络具备了提取全局依赖关系和局部特征的能力,最后采用两步训练策略优化模型训练过程.
测试集的分割结果表明,模型分割ICA区域的表现最好,推测是因为ICA区域的动脉较粗,成像清晰,更易于分割,而动脉较细的PCA区域体素强度低,容易被误判为脑组织.为进一步分析模型的泛用性,我们分别统计了模型在颅内动脉瘤患者影像和健康人影像中的动脉树区域分割表现,其中5名患者的平均Dice系数为72.50%±2.07%,而5名健康人的平均Dice系数为76.94%±2.96%,说明模型具有一定的鲁棒性,分割性能没有明显受到病灶影响.
3 结论
本文提出了基于DBCNet的TOF-MRA中脑动脉树区域自动分割方法,将TOF-MRA影像输入深度学习模型即可得到6个区域的动脉分割掩膜.实验结果显示,相较于其他先进网络,本文提出的方法在Dice系数和HD95两种指标上均取得了更优结果,输出的分割结果能够覆盖动脉树主要区域,可以辅助人工完成动脉树区域的标注.
利益冲突
无
参考文献
Vascular territorial segmentation and volumetric blood flow measurement using dynamic contrast enhanced magnetic resonance angiography of the brain
[J].
DOI:10.1177/0271678X17702394
URL
[本文引用: 1]

This study proposes a method for territorial segmentation and volumetric flow rate (VFR) distribution measurement of cerebral territories based on time-resolved contrast enhanced magnetic-resonance-angiography (MRA). The method uses an iterative region-growing algorithm based on bolus-arrival-time with increased temporal resolution. Eight territories were segmented: (1) right and (2) left internal carotid arteries, including the middle cerebral artery (ICA+MCA), excluding the anterior cerebral arteries (ACA); (3) right and left ACA (R+L-ACA); (4) right and (5) left external carotid arteries (ECA); (6) right and (7) left posterior cerebral arteries (PCA); and (8) vertebrobasilar territory. VFR percentage, relative to the entire brain (rVFR), was measured based on territorial volume as a function of time. Mean rVFR values of fifteen healthy subjects were: ICA+MCA = 23 ± 2%, R + L-ACA = 17 ± 3%, ECA = 4 ± 2%, PCA = 12 ± 2%, and vertebrobasilar territory = 31 ± 4%. Excluding the ECA-rVFR, which is underestimated, these values are comparable to previously reported values. Six subjects were scanned twice, demonstrating comparable and even higher reproducibility than previously reported using phase-contrast, yet with faster scan time (∼1 min). This method was implemented in one patient with MCA occlusion and one with Moyamoya syndrome scanned before and after bypass surgery, demonstrating its clinical potential for quantitative assessment of the degree of occlusion and the effect of surgery.
Automatic cerebrovascular segmentation methods-a review
[J].
A fast and fully automatic method for cerebrovascular segmentation on time-of-flight (TOF) MRA image
[J].
DOI:10.1007/s10278-010-9326-1
PMID:20824304
[本文引用: 1]

The precise three-dimensional (3-D) segmentation of cerebral vessels from magnetic resonance angiography (MRA) images is essential for the detection of cerebrovascular diseases (e.g., occlusion, aneurysm). The complex 3-D structure of cerebral vessels and the low contrast of thin vessels in MRA images make precise segmentation difficult. We present a fast, fully automatic segmentation algorithm based on statistical model analysis and improved curve evolution for extracting the 3-D cerebral vessels from a time-of-flight (TOF) MRA dataset. Cerebral vessels and other tissue (brain tissue, CSF, and bone) in TOF MRA dataset are modeled by Gaussian distribution and combination of Rayleigh with several Gaussian distributions separately. The region distribution combined with gradient information is used in edge-strength of curve evolution as one novel mode. This edge-strength function is able to determine the boundary of thin vessels with low contrast around brain tissue accurately and robustly. Moreover, a fast level set method is developed to implement the curve evolution to assure high efficiency of the cerebrovascular segmentation. Quantitative comparisons with 10 sets of manual segmentation results showed that the average volume sensitivity, the average branch sensitivity, and average mean absolute distance error are 93.6%, 95.98%, and 0.333 mm, respectively. By applying the algorithm to 200 clinical datasets from three hospitals, it is demonstrated that the proposed algorithm can provide good quality segmentation capable of extracting a vessel with a one-voxel diameter in less than 2 min. Its accuracy and speed make this novel algorithm more suitable for a clinical computer-aided diagnosis system.
Automatic detection for cerebral aneurysms in TOF-MRA images based on fuzzy label and deep learning
[J].
基于模糊标签和深度学习的TOF-MRA影像脑动脉瘤自动检测
[J].
Reproducibility of image-based computational models of intracranial aneurysm: a comparison between 3D rotational angiography, CT angiography and MR angiography
[J].
DOI:10.1186/s12938-016-0163-4
PMID:27150439
[本文引用: 1]

Background: Reconstruction of patient-specific biomechanical model of intracranial aneurysm has been based on different imaging modalities. However, different imaging techniques may influence the model geometry and the computational fluid dynamics (CFD) simulation. The aim of this study is to evaluate the differences of the morphological and hemodynamic parameters in the computational models reconstructed from computed tomography angiography (CTA), magnetic resonance angiography (MRA) and 3D rotational angiography (3DRA). Methods: Ten patients with cerebral aneurysms were enrolled in the study. MRA, CTA and 3DRA were performed on all patients. For each patient, three patient-specific models were reconstructed respectively based on the three sets of imaging data of the patient. CFD simulations were performed on each model. Model geometry and hemodynamic parameters were compared between the three models. Results: In terms of morphological parameters, by comparing CTA based models (CM) and 3DRA based models (DM) which were treated as the "standard models", the aspect ratio had the minimum difference (Delta = 8.3 +/- 1.72 %, P = 0.953) and the surface distance was 0.25 +/- 0.07 mm. Meanwhile, by comparing MRA based models (MM) and DM, the size had the minimum difference (Delta = 6.6 +/- 1.85 %, P = 0.683) and the surface distance was 0.36 +/- 0.1 mm. In respect of hemodynamic parameters, all three models showed a similar distribution: low average WSS at the sack, high OSI at the body and high average WSSG at the neck. However, there was a large variation in the average WSS (Delta = 34 +/- 5.13 % for CM, Delta = 40.6 +/- 9.21 % for MM). Conclusion: CTA and MRA have no significant differences in reproducing intracranial aneurysm geometry. The CFD results suggests there might be some significant differences in hemodynamic parameters between the three imaging-based models and this needs to be considered when interpreting the CFD results of different imaging-based models. If we only need to study the main flow patterns, three types of image-based model might be all suitable for patient-specific computational modeling studies.
An attention residual U-Net with differential preprocessing and geometric postprocessing: Learning how to segment vasculature including intracranial aneurysms
[J].DOI:10.1016/j.media.2022.102697 URL [本文引用: 1]
Multiple self-attention network for intracranial vessel segmentation
[C]//
Novel dataset and evaluation of state-of-the-art vessel segmentation methods
[J].
3D vessel-like structure segmentation in medical images by an edge-reinforced network
[J].DOI:10.1016/j.media.2022.102581 URL [本文引用: 1]
Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: A literature review and meta-analysis
[J].DOI:10.1002/ca.v34.7 URL [本文引用: 1]
Automatic segmentation method which divides a cerebral artery tree in time-of-flight MR-angiography into artery segments
[J].
A 3D model of human cerebrovasculature derived from 3T magnetic resonance angiography
[J].
DOI:10.1007/s12021-008-9028-8
PMID:19016001
[本文引用: 1]

The human cerebrovasculature is extremely complicated and its three dimensional (3D) highly parcellated models, though necessary, are unavailable. We constructed a digital cerebrovascular model from a high resolution, 3T 3D time-of-flight magnetic resonance angiography scan. This model contains the arterial and venous systems and is 3D, geometric, highly parcellated, fully segmented, and completely labeled with name, diameter, and variants. Our approach replaces the tedious and time consuming process of checking and correcting automatic segmentation results done at 2D image level with an aggregate and faster process at 3D model level. The creation of the vascular model required vessel pre-segmentation, centerline extraction, vascular segments connection, centerline smoothing, vessel surface construction, vessel grouping, tracking, editing, labeling, setting diameter, and checking correctness and completeness. For comparison, the same scan was segmented automatically with 59.8% sensitivity and only 16.5% of vessels smaller than 1 pixel size were extracted. To check and correct this automatic segmentation requires 8 weeks. Conversely, the speedup of our approach (the number of 2D segmented areas/the number of 3D vascular segments) is 34. This cerebrovascular model can serve as a reference framework in clinical, research, and educational applications. The wealth of information aggregated with its quantification capabilities can augment or replace numerous textbook chapters. Five applications of the vascular model were described. The model is easily extendable in content, parcellation, and labeling, and the proposed approach is applicable for building a whole body vascular system.
Quantification of morphometry and intensity features of intracranial arteries from 3D TOF MRA using the intracranial artery feature extraction (iCafe): A reproducibility study
[J].
DOI:S0730-725X(18)30538-1
PMID:30580079
[本文引用: 1]

Accurate and reliable vascular features extracted from 3D time-of-flight (TOF) magnetic resonance angiography (MRA) can help evaluate cerebral vascular diseases and conditions. The goal of this study was to evaluate the reproducibility of an intracranial artery feature extraction (iCafe) algorithm for quantitative analysis of intracranial arteries from TOF MRA.Twenty-four patients with known intracranial artery stenosis were recruited and underwent two separate MRA scans within 2 weeks of each other. Each dataset was blinded to associated imaging and clinical data and then processed independently using iCafe. Inter-scan reproducibility analysis was performed on the 24 pairs of scans while intra-/inter-operator reproducibility and stenosis detection were assessed on 8 individual MRA scans. After tracing the vessels visualized on TOF MRA, iCafe was used to automatically extract the locations with stenosis and eight other vascular features. The vascular features included the following six morphometry and two signal intensity features: artery length (total, distal, and proximal), volume, number of branches, average radius of the M1 segment of the middle cerebral artery, and average normalized intensity of all arteries and large vertical arteries. A neuroradiologist independently reviewed the images to identify locations of stenosis for the reference standard. Reproducibility of stenosis detection and vascular features was assessed using Cohen's kappa, the intra-class correlation coefficient (ICC), and within-subject coefficient of variation (CV).The segment-based sensitivity of iCafe for stenosis detection ranged from 83.3-91.7% while specificity was 97.4%. Kappa values for inter-scan and intra-operator reproducibility were 0.73 and 0.77, respectively. All vascular features demonstrated excellent inter-scan and intra-operator reproducibility (ICC = 0.91-1.00, and CV = 1.21-8.78% for all markers), and good to excellent inter-operator reproducibility (ICC = 0.76-0.99, and CV = 3.27-15.79% for all markers).Intracranial artery features can be reliably quantified from TOF MRA using iCafe to provide both clinical diagnostic assistance and facilitate future investigative quantitative analyses.Copyright © 2018 Elsevier Inc. All rights reserved.
Quantitative assessment of the intracranial vasculature in an older adult population using iCafe
[J].
DOI:S0197-4580(19)30077-6
PMID:31026623
[本文引用: 1]

Comprehensive quantification of intracranial artery features may help us assess and understand variations of blood supply during brain development and aging. We analyzed vasculature features of 163 participants (age 56-85 years, mean of 71) from a community study to investigate if any of the features varied with age. Three-dimensional time-of-flight magnetic resonance angiography images of these participants were processed in IntraCranial artery feature extraction technique (a recently developed technique to obtain quantitative features of arteries) to divide intracranial vasculatures into anatomical segments and generate 8 morphometry and intensity features for each segment. Overall, increase in age was found negatively associated with number of branches and average order of intracranial arteries while positively associated with tortuosity, which remained after adjusting for cardiovascular risk factors. The associations with number of branches and average order were consistently found between 3 main intracranial artery regions, whereas the association with tortuosity appeared to be present only in middle cerebral artery/distal arteries. The combination of time-of-flight magnetic resonance angiography and IntraCranial artery feature extraction technique may provide an effective way to study vascular conditions and changes in the aging brain.Copyright © 2019 Elsevier Inc. All rights reserved.
A survey on U-shaped networks in medical image segmentations
[J].DOI:10.1016/j.neucom.2020.05.070 URL [本文引用: 2]
Segmentation of breast tumors based on fully convolutional network and dynamic contrast enhanced magnetic resonance image
[J].
基于全卷积网络的乳腺肿瘤动态增强磁共振图像分割
[J].
Camouflaged object detection
[C]//
BiconNet: An edge-preserved connectivity-based approach for salient object detection
[J].DOI:10.1016/j.patcog.2021.108231 URL [本文引用: 1]
Swin transformer: Hierarchical vision transformer using shifted windows
[C]// International Conference on Computer Vision,
User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability
[J].
DOI:10.1016/j.neuroimage.2006.01.015
PMID:16545965
[本文引用: 1]

Active contour segmentation and its robust implementation using level set methods are well-established theoretical approaches that have been studied thoroughly in the image analysis literature. Despite the existence of these powerful segmentation methods, the needs of clinical research continue to be fulfilled, to a large extent, using slice-by-slice manual tracing. To bridge the gap between methodological advances and clinical routine, we developed an open source application called ITK-SNAP, which is intended to make level set segmentation easily accessible to a wide range of users, including those with little or no mathematical expertise. This paper describes the methods and software engineering philosophy behind this new tool and provides the results of validation experiments performed in the context of an ongoing child autism neuroimaging study. The validation establishes SNAP intrarater and interrater reliability and overlap error statistics for the caudate nucleus and finds that SNAP is a highly reliable and efficient alternative to manual tracing. Analogous results for lateral ventricle segmentation are provided.
Aneurysms of the distal anterior cerebral artery: report of 14 cases and a review of the literature
[J].Distal anterior cerebral artery aneurysms are rare and compose about 4.5% of all intracranial aneurysms. They generally arise at the bifurcation of the pericallosal and callosomarginal arteries. Their surgical approach is different from those of other anterior circulation aneurysms. These aneurysms present some special difficulties for neurosurgeons, including narrow exposure in the interhemispheric fissure, dense adhesions between the cingulate gyri, difficulty in controlling the parent artery, and the association of multiple aneurysms and vascular anomalies.Between January 1975 and May 1996, 14 cases of saccular aneurysms of the distal anterior cerebral artery were operated at the University of Hacettepe. The clinical presentations, neuroradiological findings, and operative approaches of these aneurysms were analyzed. In addition, the clinical series and isolated case reports in the English literature were also extensively reviewed.The incidence of the aneurysms in this location was 2.8% of a total of 494 surgically treated cases in our center. Of 14 patients, eight were women and six were men. Multiple aneurysms were found in five patients (35%). All patients were operated via the interhemispheric route. Thirteen patients had good outcome and one patient died.We believe that all difficulties related to distal anterior cerebral artery aneurysms can be minimized with sufficient knowledge of microsurgery and surgical anatomy, using microtechniques and experience.
A computational approach to edge detection
[J].
Deep residual learning for image recognition
[C]//
CBAM: Convolutional block attention module
[J].
Unified focal loss: Generalising dice and cross entropy-based losses to handle class imbalanced medical image segmentation
[J].DOI:10.1016/j.compmedimag.2021.102026 URL [本文引用: 2]
nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation
[J].
DOI:10.1038/s41592-020-01008-z
PMID:33288961
[本文引用: 1]

Biomedical imaging is a driver of scientific discovery and a core component of medical care and is being stimulated by the field of deep learning. While semantic segmentation algorithms enable image analysis and quantification in many applications, the design of respective specialized solutions is non-trivial and highly dependent on dataset properties and hardware conditions. We developed nnU-Net, a deep learning-based segmentation method that automatically configures itself, including preprocessing, network architecture, training and post-processing for any new task. The key design choices in this process are modeled as a set of fixed parameters, interdependent rules and empirical decisions. Without manual intervention, nnU-Net surpasses most existing approaches, including highly specialized solutions on 23 public datasets used in international biomedical segmentation competitions. We make nnU-Net publicly available as an out-of-the-box tool, rendering state-of-the-art segmentation accessible to a broad audience by requiring neither expert knowledge nor computing resources beyond standard network training.
Brain tumor segmentation and radiomics survival prediction: Contribution to the BRATS 2017 Challenge
[J].
V-Net: Fully convolutional neural networks for volumetric medical image segmentation
[C]//
3D U-Net: Learning dense volumetric segmentation from sparse annotation
[C]//
/
| 〈 |
|
〉 |