
波谱学杂志 ›› 1989, Vol. 6 ›› Issue (1): 47-52.
孙琼丽1, 丛爱真1, 黄新1, 余秀芬2, 黄种乐2
Sun Qiongli1, Cong Aizhen1, Huang Xin1
摘要: 在室温、77K条件下,对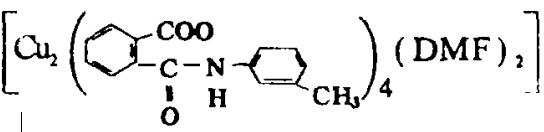 ·4DMF(1)簇合物的固态和溶液样品进行了EPR谱的测定,获得三套谱图(Ⅰ、Ⅱ、Ⅲ),其分别归属于簇合物中未配对电子的两种形式:(1)类似于自由的单铜离子的未配对电了(Ⅰ、Ⅱ两套谱).(2)双铜未配对电子偶合成的三重态(Ⅲ套谱).
·4DMF(1)簇合物的固态和溶液样品进行了EPR谱的测定,获得三套谱图(Ⅰ、Ⅱ、Ⅲ),其分别归属于簇合物中未配对电子的两种形式:(1)类似于自由的单铜离子的未配对电了(Ⅰ、Ⅱ两套谱).(2)双铜未配对电子偶合成的三重态(Ⅲ套谱).
文中用双铜的三重态自旋哈密顿HS=βHgS+DSz2+E(Sx2-Sy2)-(2)/3D公式计算三重态EPR谱的参数.
题目化合物(1)与双铜簇合物 ·4DMF(2)相比较,在配体结构上稍有不同(前者,甲苯胺中的甲基是连接于苯环的间位;而后者,甲基是连接于苯环的对位),由此引起一些磁性参数:有效磁矩μeff、磁交换相互作用参数J、相对的电子自旋浓度ρ和EPR谱的超精细结构(h.f.s)参数都有所不同.
·4DMF(2)相比较,在配体结构上稍有不同(前者,甲苯胺中的甲基是连接于苯环的间位;而后者,甲基是连接于苯环的对位),由此引起一些磁性参数:有效磁矩μeff、磁交换相互作用参数J、相对的电子自旋浓度ρ和EPR谱的超精细结构(h.f.s)参数都有所不同.