
- Jul. 12, 2025
- Home
- About Us
- Editorial Board
- Instruction
- Subscription
- Advertisement
- Contact Us
- Chinese
- RSS

Chinese Journal of Magnetic Resonance ›› 2021, Vol. 38 ›› Issue (4): 571-584.doi: 10.11938/cjmr20212926
Yao XIAO1,2,Chang-jiu XIA3,Xian-feng YI1,*( ),Feng-qing LIU1,2,Shang-bin LIU4,An-min ZHENG1,*(
),Feng-qing LIU1,2,Shang-bin LIU4,An-min ZHENG1,*( )
)
Received:2021-06-28
Online:2021-12-05
Published:2021-08-14
Contact:
Xian-feng YI,An-min ZHENG
E-mail:yxf@wipm.ac.cn;zhenganm@wipm.ac.cn
CLC Number:
Yao XIAO,Chang-jiu XIA,Xian-feng YI,Feng-qing LIU,Shang-bin LIU,An-min ZHENG. Progress in the Studies on Sn-Zeolites by Solid-State Nuclear Magnetic Resonance[J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 571-584.
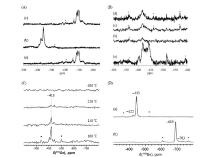
Fig.1
(A) 119Sn MAS NMR spectra of Sn-Beta after different treatments: (a) Calcined; (b) Dehydrated after calcination; (c) Rehydrated after step (b). (B) (a) 119Sn MAS and (b~d) 1H-119Sn CP MAS NMR spectra of dehydrated Sn-Beta. The CP contact times from 1H to 119Sn were varied: (b) 0.2 ms, (c) 1.0 ms, (d) 2.0 ms. (C) 1H-119Sn CP-CPMG MAS NMR spectra of 119Sn-Beta sample dehydrated under vacuum conditions at different temperatures. (D) 119Sn DP-CPMG MAS NMR spectra of (a) dehydrated and (b) hydrated Sn-Beta. The spinning sidebands are marked as asterisks (Reproduced from Refs. [6] and [26])
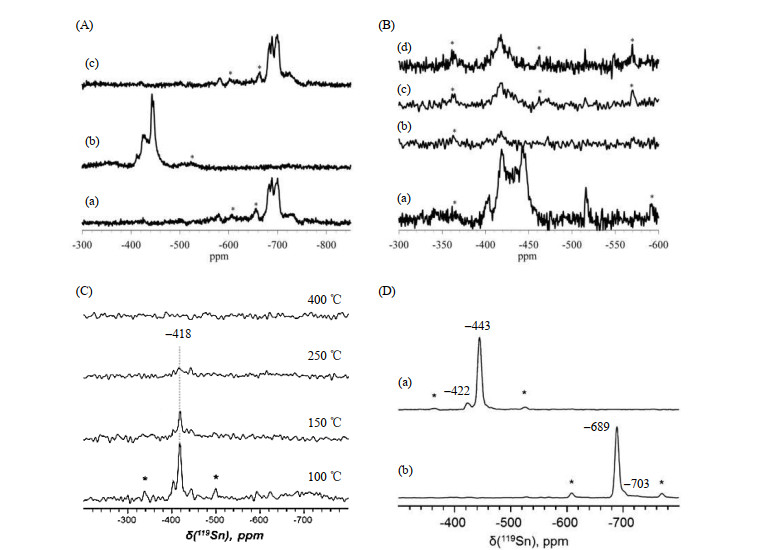

Fig.2
(a) Variation of 119Sn DP-CPMG MAS NMR spectra of 119Sn-Beta with time after exposure to water (H2O/Sn=4); (b) Variation of the normalized integrated intensity of 119Sn MAS NMR signals at ca. ?581, ?689, and ?703 ppm with time of exposure to water; (c) Dissociative adsorption scheme of water over tin sites in Sn-Beta (Reproduced from Ref. [26])
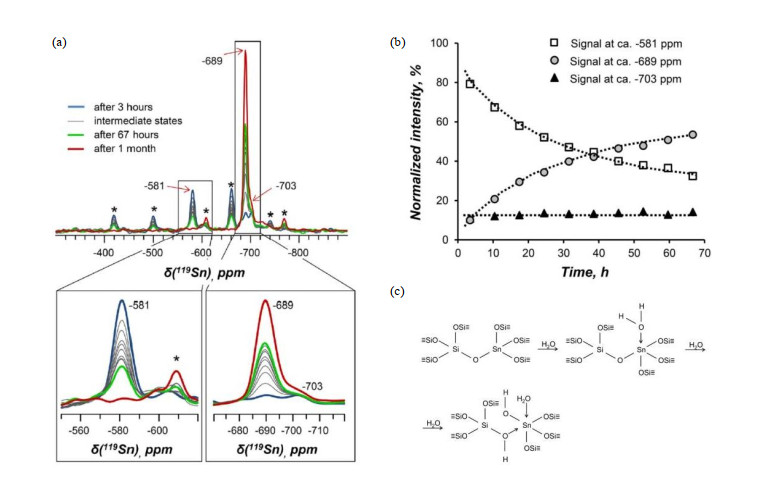

Fig.3
(a) 1H MAS NMR spectra of dehydrated Sn-Beta before and after exposure to water vapors for 70 h; (b) Time-resolved 1H MAS NMR spectra acquired during the hydration of Sn-Beta; (c) Normalized intensities of 1H and 119Sn MAS NMR signals vs. hydration time; (d, e) Two different hydration mechanism in dehydrated Sn-Beta (Reproduced from Ref. [27])

Fig.7
Site time yield (STY) for (a) glucose isomerization in water and (b) aldol condensation of benzaldehyde and acetone in toluene catalyzed by different Sn-Beta catalysts plotted against the percent integrated 31P peak area normalized by P and Sn content at (a) δiso = 55.8 and 54.9 ppm and (b) δiso = 58.6 and 57.2 ppm (Reproduced from Ref. [45])
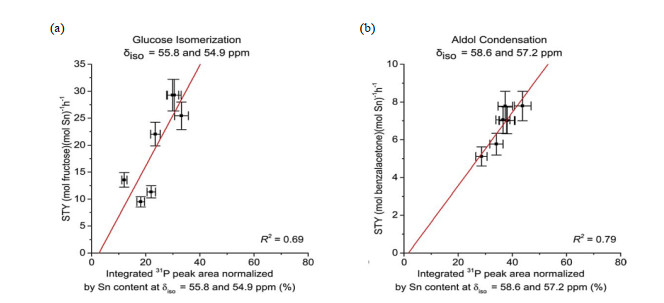

Fig.8
(a) 31P MAS and (b) 13C CP MAS NMR spectra of Si-Beta and Sn-containing Beta zeolites recorded after adsorption of TMPO and 2-13C-acetone, respectively. (c) 1H MAS NMR or difference spectra of various dehydrated Sn-containing Beta zeolites recorded before and after adsorption of ammonia (Reproduced from Ref. [46])
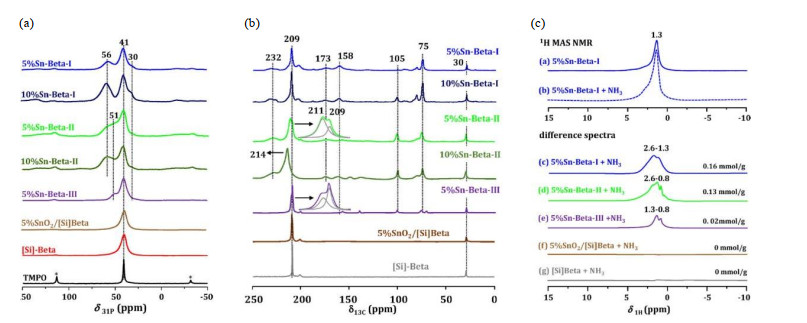

Fig.11
(A) 13C MAS NMR of (a) glucose adsorbed into Si-Beta, (b) fructose adsorbed into Si-Beta, (c) glucose adsorbed into Sn-Beta, (d) fructose adsorbed into Sn-Beta. (B) Spectra from fructose adsorbed into Sn-Beta: (a) CP contact time of 0.1 ms; (b) CP contact time of 1.0 ms; and (c) no CP (Reproduced from Ref. [6])
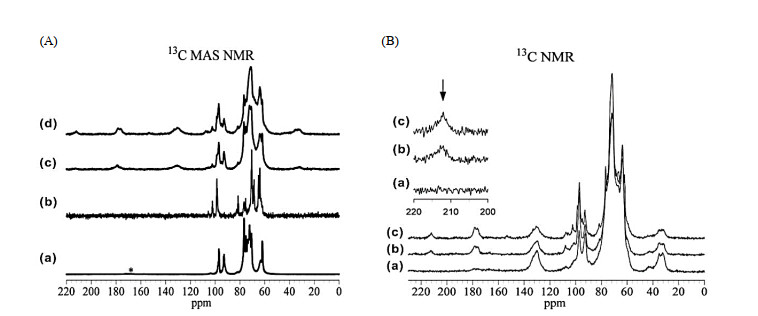
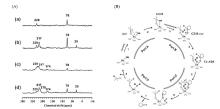
Fig.12
(A) 13C CP MAS NMR spectra of (a) 30 μmol⋅g?1 and (b) 100 μmol⋅g?1 of 2-13C-acetone adsorbed on Sn-Beta zeolite dehydrated at 393 K, (c) 30 μmol⋅g?1 and (d) 100 μmol⋅g?1 of 2-13C-acetone adsorbed on Sn-Beta zeolite dehydrated at 673 K. (B) Proposed catalytic cycle for the reaction between acetone and cyclohexanol on Sn-Beta zeolite. (Reproduced from Ref. [50])

| 1 |
ROGELJ J , NABEL J , CHEN C , et al. Copenhagen accord pledges are paltry[J]. Nature, 2010, 464 (7292): 1126- 1128.
doi: 10.1038/4641126a |
| 2 |
RAGAUSKAS A J , WILLIAMS C K , DAVISON B H , et al. The path forward for biofuels and biomaterials[J]. Science, 2006, 311 (5760): 484- 489.
doi: 10.1126/science.1114736 |
| 3 |
ALVIRA P , TOMáS-PEJó E , BALLESTEROS M , et al. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review[J]. Bioresource Technol, 2010, 101 (13): 4851- 4861.
doi: 10.1016/j.biortech.2009.11.093 |
| 4 |
MOLINER M , ROMáN-LESHKOV Y , DAVIS M E . Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water[J]. Proc Natl Acad Sci USA, 2010, 107 (14): 6164- 6168.
doi: 10.1073/pnas.1002358107 |
| 5 |
ROMáN-LESHKOV Y , MOLINER M , LABINGER J A , et al. Mechanism of glucose isomerization using a solid Lewis acid catalyst in water[J]. Angew Chem Int Ed, 2010, 49 (47): 8954- 8957.
doi: 10.1002/anie.201004689 |
| 6 |
BERMEJO-DEVAL R , ASSARY R S , NIKOLLA E , et al. Metalloenzyme-like catalyzed isomerizations of sugars by Lewis acid zeolites[J]. Proc Natl Acad Sci USA, 2012, 109 (25): 9727- 9732.
doi: 10.1073/pnas.1206708109 |
| 7 |
NIKOLLA E , ROMáN-LESHKOV Y , MOLINER M , et al. "One-Pot" synthesis of 5-(hydroxymethyl)furfural from carbohydrates using tin-Beta zeolite[J]. ACS Catal, 2011, 1 (4): 408- 410.
doi: 10.1021/cs2000544 |
| 8 |
HOLM M S , SARAVANAMURUGAN S , TAARNING E . Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts[J]. Science, 2010, 328 (5978): 602- 605.
doi: 10.1126/science.1183990 |
| 9 |
ZHANG X , WILSON K , LEE A F . Heterogeneously catalyzed hydrothermal processing of C5-C6 sugars[J]. Chem Rev, 2016, 116 (19): 12328- 12368.
doi: 10.1021/acs.chemrev.6b00311 |
| 10 |
MIKA L T , CSEFALVAY E , NEMETH A . Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability[J]. Chem Rev, 2018, 118 (2): 505- 613.
doi: 10.1021/acs.chemrev.7b00395 |
| 11 |
BORONAT M , CORMA A , RENZ M . Mechanism of the Meerwein-Ponndorf-Verley-Oppenauer (MPVO) redox equilibrium on Sn- and Zr-Beta zeolite catalysts[J]. J Phys Chem B, 2006, 110 (42): 21168- 21174.
doi: 10.1021/jp063249x |
| 12 |
CORMA A , DOMINE M E , VALENCIA S . Water-resistant solid Lewis acid catalysts: Meerwein-Ponndorf-Verley and Oppenauer reactions catalyzed by tin-beta zeolite[J]. J Catal, 2003, 215 (2): 294- 304.
doi: 10.1016/S0021-9517(03)00014-9 |
| 13 |
LIU Y J , XIAO Y , XIA C J , et al. Insight into the effects of acid characteristics on the catalytic performance of Sn-MFI zeolites in the transformation of dihydroxyacetone to methyl lactate[J]. J Catal, 2020, 391, 386- 396.
doi: 10.1016/j.jcat.2020.09.004 |
| 14 |
CORMA A , NEMETH L T , RENZ M , et al. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations[J]. Nature, 2001, 412 (6845): 423- 425.
doi: 10.1038/35086546 |
| 15 |
ZHENG A M , LI S H , LIU S B , et al. Acidic properties and structure-activity correlations of solid acid catalysts revealed by solid-state NMR spectroscopy[J]. Acc Chem Res, 2016, 49 (4): 655- 663.
doi: 10.1021/acs.accounts.6b00007 |
| 16 |
KLINOWSKI J . Solid-state NMR studies of molecular sieve catalysts[J]. Chem Rev, 1991, 91 (7): 1459- 1479.
doi: 10.1021/cr00007a010 |
| 17 |
JIANG Y J , HUANG J , DAI W L , et al. Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts[J]. Solid State Nucl Magn Reson, 2011, 39 (3-4): 116- 141.
doi: 10.1016/j.ssnmr.2011.03.007 |
| 18 |
WANG Z C , JIANG Y J , BAIKER A , et al. Pentacoordinated aluminum species: New frontier for tailoring acidity-enhanced silica-alumina catalysts[J]. Acc Chem Res, 2020, 53 (11): 2648- 2658.
doi: 10.1021/acs.accounts.0c00459 |
| 19 |
SUN T T , XU S T , XIAO D , et al. Water-induced structural dynamic process in molecular sieves under mild hydrothermal conditions: Ship-in-a-bottle strategy for acidity identification and catalyst modification[J]. Angew Chem, 2020, 132 (46): 20853- 20862.
doi: 10.1002/ange.202009648 |
| 20 |
GONG K , LIU Z M , LIANG L X , et al. Acidity and local confinement effect in mordenite probed by solid-state NMR spectroscopy[J]. J Phys Chem Lett, 2021, 12 (9): 2413- 2422.
doi: 10.1021/acs.jpclett.0c03610 |
| 21 |
ZHENG A M , LIU S B , DENG F . 31P NMR chemical shifts of phosphorus probes as reliable and practical acidity scales for solid and liquid catalysts[J]. Chem Rev, 2017, 117 (19): 12475- 12531.
doi: 10.1021/acs.chemrev.7b00289 |
| 22 |
YI X , PENG Y K , CHEN W , et al. Surface fingerprinting of faceted metal oxides and porous zeolite catalysts by probe-assisted solid-state NMR approaches[J]. Acc Chem Res, 2021, 54 (10): 2421- 2433.
doi: 10.1021/acs.accounts.1c00069 |
| 23 |
XU S T , ZHENG A M , WEI Y X , et al. Direct observation of cyclic carbenium Ions and their role in the catalytic cycle of the methanol-to-olefin reaction over chabazite zeolites[J]. Angew Chem Int Ed, 2013, 52 (44): 11564- 11568.
doi: 10.1002/anie.201303586 |
| 24 |
WOLF P , VALLA M , ROSSINI A J , et al. NMR signatures of the active sites in Sn-β zeolite[J]. Angew Chem, 2014, 126 (38): 10343- 10347.
doi: 10.1002/ange.201403905 |
| 25 |
KOLYAGIN Y G , YAKIMOV A V , TOLBORG S , et al. Application of 119Sn CPMG MAS NMR for fast characterization of Sn sites in zeolites with natural 119Sn isotope abundance[J]. J Phys Chem Lett, 2016, 7 (7): 1249- 1253.
doi: 10.1021/acs.jpclett.6b00249 |
| 26 |
YAKIMOV A V , KOLYAGIN Y G , TOLBORG S , et al. 119Sn MAS NMR study of the interaction of probe molecules with Sn-BEA: The origin of penta- and hexacoordinated tin formation[J]. J Phys Chem C, 2016, 120 (49): 28083- 28092.
doi: 10.1021/acs.jpcc.6b09999 |
| 27 |
SUSHKEVICH V L , KOTS P A , KOLYAGIN Y G , et al. Origin of water-induced Brønsted acid sites in Sn-BEA zeolites[J]. J Phys Chem C, 2019, 123 (9): 5540- 5548.
doi: 10.1021/acs.jpcc.8b12462 |
| 28 |
WOLF P , VALLA M , NúñEZ-ZARUR F , et al. Correlating synthetic methods, morphology, atomic-level structure, and catalytic activity of Sn-β catalysts[J]. ACS Catal, 2016, 6 (7): 4047- 4063.
doi: 10.1021/acscatal.6b00114 |
| 29 |
ROY S , BAKHMUTSKY K , MAHMOUD E , et al. Probing Lewis acid sites in Sn-Beta zeolite[J]. ACS Catal, 2013, 3 (4): 573- 580.
doi: 10.1021/cs300599z |
| 30 |
KOLYAGIN Y G , YAKIMOV A V , TOLBORG S , et al. Direct observation of tin in different T-sites of Sn-BEA by one- and two-dimensional 119Sn MAS NMR spectroscopy[J]. J Phys Chem Lett, 2018, 9 (13): 3738- 3743.
doi: 10.1021/acs.jpclett.8b01415 |
| 31 |
WOLF P , LIAO W C , ONG T C , et al. Identifying Sn site heterogeneities prevalent among Sn-Beta zeolites[J]. Helvetica Chimica Acta, 2016, 99 (12): 916- 927.
doi: 10.1002/hlca.201600234 |
| 32 |
GUNTHER W R , MICHAELIS V K , CAPORINI M A , et al. Dynamic nuclear polarization NMR enables the analysis of Sn-Beta zeolite prepared with natural abundance 119Sn precursors[J]. J Am Chem Soc, 2014, 136 (17): 6219- 6222.
doi: 10.1021/ja502113d |
| 33 |
DIJKMANS J , DUSSELIER M , GABRIëLS D , et al. Cooperative catalysis for multistep biomass conversion with Sn/Al Beta zeolite[J]. ACS Catal, 2015, 5 (2): 928- 940.
doi: 10.1021/cs501388e |
| 34 |
OVERHAUSER A W . Polarization of nuclei in metals[J]. Phys Rev, 1953, 92 (2): 411- 415.
doi: 10.1103/PhysRev.92.411 |
| 35 |
BARKER W A . Dynamic nuclear polarization[J]. Rev Mod Phys, 1962, 34 (2): 173- 185.
doi: 10.1103/RevModPhys.34.173 |
| 36 |
QI G D , WANG Q , XU J , et al. Direct observation of tin sites and their reversible interconversion in zeolites by solid-state NMR spectroscopy[J]. Commun Chem, 2018, 1 (1): 22.
doi: 10.1038/s42004-018-0023-1 |
| 37 |
ZHAO S F , HE S L , DU KIM K , et al. Influence of hierarchical ZSM-5 catalysts with various acidity on the dehydration of glycerol to acrolein[J]. Magn Reson Lett, 2021, 1 (1): 71- 80.
doi: 10.1016/j.mrl.2021.100002 |
| 38 |
LUNSFORD J L , ROTHWELL W P , SHEN W . Acid sites in zeolite Y: A solid-state NMR and infrared study using trimethylphosphine as a probe molecule[J]. J Am Chem Soc, 1985, 107 (6): 1540- 1547.
doi: 10.1021/ja00292a015 |
| 39 |
TRICKETT C A , OSBORN-POPP T M , SU J , et al. Identification of the strong Brønsted acid site in a metal-organic framework solid acid catalyst[J]. Nat Chem, 2019, 11, 170- 176.
doi: 10.1038/s41557-018-0171-z |
| 40 | GAO X Z , ZHANG Y , WANG X M , et al. Structure and acidity changes in ultra-stable Y zeolites during hydrothermal aging: A solid-state NMR spectroscopy study[J]. Chinese J Magn Reson, 2020, 37 (1): 95- 103. |
| 高秀枝, 张翊, 王秀梅, 等. NMR研究超稳Y分子筛水热老化过程中结构与酸性的变化[J]. 波谱学杂志, 2020, 37 (1): 95- 103. | |
| 41 | XU C , CAI Z , WANG Q , et al. Preparation of biodiesel using silver-modified phosphotungstic acid as catalyst[J]. Chinese J Magn Reson, 2020, 37 (1): 86- 94. |
| 徐超, 蔡哲, 王晴, 等. 银改性磷钨酸催化制备生物柴油工艺研究[J]. 波谱学杂志, 2020, 37 (1): 86- 94. | |
| 42 |
YI X F , LIU K Y , CHEN W , et al. Origin and structural characteristics of tri-coordinated extra-framework aluminum species in dealuminated zeolites[J]. J Am Chem Soc, 2018, 140 (34): 10764- 10774.
doi: 10.1021/jacs.8b04819 |
| 43 |
YI X F , XIAO Y , LI G C , et al. From one to two: Acidic proton spatial networks in porous zeolite materials[J]. Chem Mater, 2020, 32 (3): 1332- 1342.
doi: 10.1021/acs.chemmater.0c00005 |
| 44 |
YI X F , KO H , DENG F , et al. Solid-state 31P NMR mapping of active centers and relevant spatial correlations in solid acid catalysts[J]. Nat Protoc, 2020, 15, 3527- 3555.
doi: 10.1038/s41596-020-0385-6 |
| 45 |
LEWIS J D , HA M , LUO H , et al. Distinguishing active site identity in Sn-Beta zeolites using 31P MAS NMR of adsorbed trimethylphosphine oxide[J]. ACS Catal, 2018, 8 (4): 3076- 3086.
doi: 10.1021/acscatal.7b03533 |
| 46 |
DAI W L , LEI Q F , WU G J , et al. Spectroscopic signature of Lewis acidic framework and extraframework Sn sites in Beta zeolites[J]. ACS Catal, 2020, 10 (23): 14135- 14146.
doi: 10.1021/acscatal.0c02356 |
| 47 |
KIM K D , WANG Z C , JIANG Y J , et al. The cooperative effect of Lewis and Brønsted acid sites on Sn-MCM-41 catalysts for the conversion of 1, 3-dihydroxyacetone to ethyl lactate[J]. Green Chem, 2019, 21 (12): 3383- 3393.
doi: 10.1039/C9GC00820A |
| 48 |
GUNTHER W R , MICHAELIS V K , GRIFFIN R G , et al. Interrogating the Lewis acidity of metal sites in Beta zeolites with 15N pyridine adsorption coupled with MAS NMR spectroscopy[J]. J Phys Chem C, 2016, 120 (50): 28533- 28544.
doi: 10.1021/acs.jpcc.6b07811 |
| 49 |
HARRIS J W , LIAO W C , DI IORIO J R , et al. Molecular structure and confining environment of Sn sites in single-site chabazite zeolites[J]. Chem Mater, 2017, 29 (20): 8824- 8837.
doi: 10.1021/acs.chemmater.7b03209 |
| 50 |
QI G D , CHU Y Y , WANG Q , et al. Gem-Diol-type intermediate in the activation of a ketone on Sn-β zeolite as studied by solid-state NMR spectroscopy[J]. Angew Chem, 2020, 59 (44): 19532- 19538.
doi: 10.1002/anie.202005589 |
| [1] | Yong-xiang WANG,Qiang WANG,Jun XU,Qing-hua XIA,Feng DENG. The Effects of Ammonium Hexafluorosilicate Post-Treatment on the Acidity of H-ZSM-5 Zeolite Studied by Solid-State NMR Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 514-522. |
| [2] | Zi-chun WANG,Jun HUANG,Yi-jiao JIANG. Solid-State NMR Spectroscopy Studies of Enhanced Acidity of Silica-Aluminas Based on Penta-Coordinated Aluminum Species [J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 552-570. |
| [3] | Shu-shu GAO,Shu-tao XU,Ying-xu WEI,Zhong-min LIU. Applications of Solid-State Nuclear Magnetic Resonance Spectroscopy in Methanol-to-Olefins Reaction [J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 433-447. |
| [4] | Xin CHEN,Ying-yi FU,Bin YUE,He-yong HE. Acidity and Basicity of Solid Acid Catalysts Studied by Solid-State NMR [J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 491-502. |
| [5] | LI Bo-jie,XU Jun*,WANG Qiang,WANG Xiu-mei,QI Guo-dong,DENG Feng*. Carbonylation of Methanol on Cu-H-MOR Zeolites: Insights from Solid-State NMR Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2014, 31(3): 331-340. |
| [6] | YU Zhi-Wu, ZHENG An-Min, WANG Qiang, HUANG Shing-Jong, DENG Feng, LIU Shang-Bin. Acidity Characterization of Solid Acid Catalysts by Solid- State NMR Spectroscopy: A Review on Recent Progresses [J]. Chinese Journal of Magnetic Resonance, 2010, 27(4): 485-515. |
| [7] | LI Fang1,3;ZHAO Ying-sheng2;LEI Ai-wen2;LIU Mai-li1*. Palladium Catalyzed Csp-Csp3 Bond Formation Reaction Studied by 13C NMR [J]. Chinese Journal of Magnetic Resonance, 2008, 25(4): 461-469. |
| [8] |
YANG Ying1; YAN Bao-zhen1; NIE Zhou2; WANG Mei1; TIAN Qiu2; LIU Yang2* . The Mechanism of a Reaction between α-Tocopherol and DPPH·Studied by NMR and ESR Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2008, 25(3): 331-336. |
| [9] | YE Jian-Liang, ZHENG Fa-Kun, CHEN Zhong, XIE Hong-Chen, CHEN Zhi-Wei, HUANG Pei-Qiang. 51V NMR STUDIES ON THE REACTION OF [VS4Cun](n=3-6) CLUSTERS [J]. Chinese Journal of Magnetic Resonance, 2001, 18(4): 315-320. |
| [10] | SHAO Rong-rong, HU Hong-yu, CHEN Yun-neng, SONG Guo-qiang. 1H NMR RESONANCE ASSIGNMENT OF SMALL VOLUME PEPTIDE OVARC [J]. Chinese Journal of Magnetic Resonance, 2001, 18(3): 235-241. |
| [11] | Cao Rong, Wu Daxu, Xie Xiulan, Hong Maochun, Jiang Feilong, Liu Hanqin. STUDIES ON THE NUCLEAR MA GNETIC RESONANCE OF MOLYBDENUM (TUNGSTEN)-COPPER-SULPHUR-DIALKYLDI-THIOCARBAMATE CLUSTER COMPOUNDS [J]. Chinese Journal of Magnetic Resonance, 1995, 12(1): 13-21. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 358
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 175
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||