
- Nov. 11, 2025
- Home
- About Us
- Editorial Board
- Instruction
- Subscription
- Advertisement
- Contact Us
- Chinese
- RSS

Chinese Journal of Magnetic Resonance ›› 2021, Vol. 38 ›› Issue (4): 433-447.doi: 10.11938/cjmr20212938
Previous Articles Next Articles
Shu-shu GAO1,2,Shu-tao XU1,*( ),Ying-xu WEI1,Zhong-min LIU1
),Ying-xu WEI1,Zhong-min LIU1
Received:2021-07-21
Published:2021-12-05
Online:2021-09-14
Contact:
Shu-tao XU
E-mail:xushutao@dicp.ac.cn
CLC Number:
Shu-shu GAO,Shu-tao XU,Ying-xu WEI,Zhong-min LIU. Applications of Solid-State Nuclear Magnetic Resonance Spectroscopy in Methanol-to-Olefins Reaction[J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 433-447.

Fig.2
(a) In situ solid-state 13C MAS NMR spectra of continuous-flow 13C-methanol conversion over H-SSZ-13 catalyst at 220 ℃; (b) The 13C CP/MAS NMR and (c) 2D 13C-13C CORD (COmbined R2 Driven) spin diffusion MAS NMR spectra of in situ 13C-methanol conversion over H-SSZ-13 catalyst at 220 ℃ for 30 min[39]
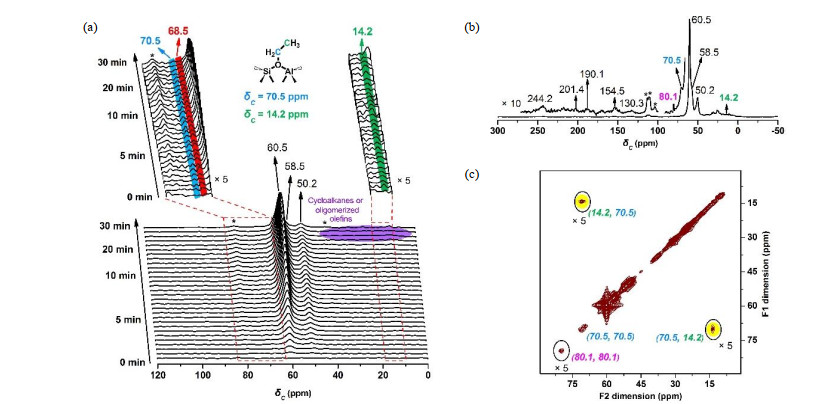
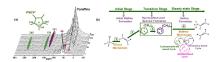
Fig.4
(a) In situ 13C MAS NMR spectra of 13CH3OH reaction over H-SSZ-13 catalyst at 275 ℃, the characteristic signals at 154 and 245 ppm illustrate the formation of pentamethylcyclopentenyl cation (PMCP+), 0~40 ppm are assigned to the paraffin; (b) ① The initial ethene is generated from methanol by direct mechanism; ② MCP species are produced from initial ethene; ③ PMBs species are produced from the co-reaction of MCP species and methanol; ④ The olefins are formed via indirect mechanism including of aromatics-based, alkenes-based, and cyclopentadienes based cycles[47]
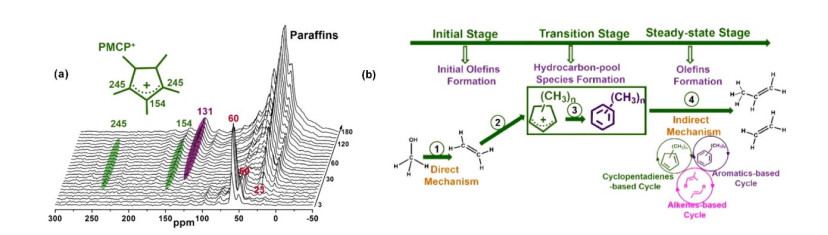

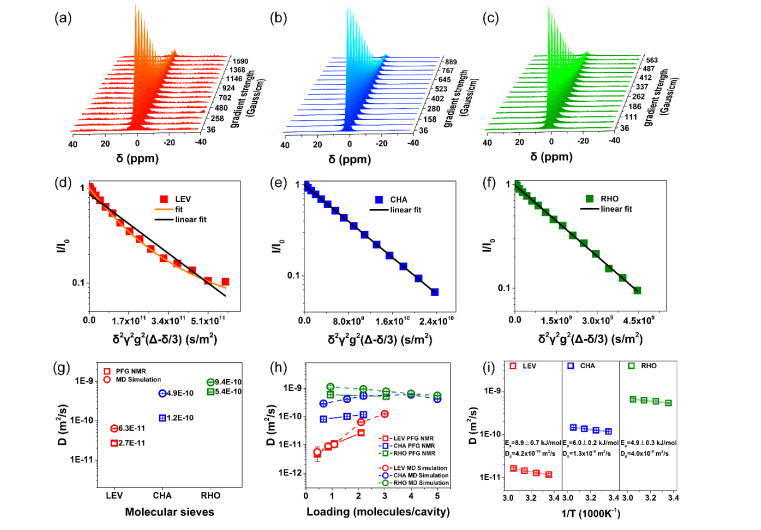
Fig.6
(a~c) 1H PFG NMR signals decay with linearly increasing gradient magnetic field strength in 16 steps for SAPO-35 (a), SAPO-34 (b) and DNL-6 (c) measured at 298 K. (d~f) The corresponding spin echo attention of PFG NMR on the log-linear scale for SAPO-35 (d), SAPO-34 (e) and DNL-6 (f) measured at 298 K. (g) The self-diffusion coefficients of methane acquired by PFG NMR and MD; (h) The loading dependence of experimental and simulated self-diffusion coefficients for methane; (i) The temperature dependence of self-diffusion coefficients for methane[68]
| 1 |
CHANG C D , SILVESTRI A J . The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts[J]. J Catal, 1977, 47 (2): 249- 259.
doi: 10.1016/0021-9517(77)90172-5 |
| 2 |
TIAN P , WEI Y X , YE M , et al. Methanol to olefins (MTO): From fundamentals to commercialization[J]. ACS Catal, 2015, 5 (3): 1922- 1938.
doi: 10.1021/acscatal.5b00007 |
| 3 |
DUSSELIER M , DAVIS M E . Small-pore zeolites: Synthesis and catalysis[J]. Chem Rev, 2018, 118 (11): 5265- 5329.
doi: 10.1021/acs.chemrev.7b00738 |
| 4 |
MOLINER M , MARTíNEZ C , CORMA A . Synthesis strategies for preparing useful small pore zeolites and zeotypes for gas separations and catalysis[J]. Chem Mater, 2014, 26 (1): 246- 258.
doi: 10.1021/cm4015095 |
| 5 |
XU S T , ZHENG A M , WEI Y X , et al. Direct observation of cyclic carbenium ions and their role in the catalytic cycle of the methanol-to-olefin reaction over chabazite zeolites[J]. Angew Chem Int Ed, 2013, 52 (44): 11564- 11568.
doi: 10.1002/anie.201303586 |
| 6 |
XU J , WANG Q , DENG F . Metal active sites and their catalytic functions in zeolites: Insights from solid-state NMR spectroscopy[J]. Acc Chem Res, 2019, 52 (8): 2179- 2189.
doi: 10.1021/acs.accounts.9b00125 |
| 7 |
ZHANG W P , XU S T , HAN X W , et al. In situ solid-state NMR for heterogeneous catalysis: a joint experimental and theoretical approach[J]. Chem Soc Rev, 2012, 41 (1): 192- 210.
doi: 10.1039/C1CS15009J |
| 8 |
YI X F , LIU K Y , CHEN W , et al. Origin and structural characteristics of tri-coordinated extra-framework aluminum species in dealuminated zeolites[J]. J Am Chem Soc, 2018, 140 (34): 10764- 10774.
doi: 10.1021/jacs.8b04819 |
| 9 | LIN Z Y , HUO H , WANG Q H , et al. Progress in solid-state NMR studies of monoclinic lithium vanadium phosphate[J]. Chinese J Magn Reson, 2020, 37 (1): 16- 27. |
| 林泽羽, 霍华, 王启航, 等. 固体核磁共振研究单斜磷酸钒锂的进展[J]. 波谱学杂志, 2020, 37 (1): 16- 27. | |
| 10 | GAO X Z , ZHANG Y , WANG X M , et al. Structure and acidity changes in ultra-stable Y zeolites during hydrothermal aging: A solid-state NMR spectroscopy study NMR[J]. Chinese J Magn Reson, 2020, 37 (1): 95- 103. |
| 高秀枝, 张翊, 王秀梅, 等. 研究超稳Y分子筛水热老化过程中结构与酸性的变化[J]. 波谱学杂志, 2020, 37 (1): 95- 103. | |
| 11 |
CHEN N Y , REAGAN W J . Evidence of autocatalysis in methanol to hydrocarbon reactions over zeolite catalysts[J]. J Catal, 1979, 59 (1): 123- 129.
doi: 10.1016/S0021-9517(79)80050-0 |
| 12 |
ONO Y , MORI T . Mechanism of methanol conversion into hydrocarbons over ZSM-5 zeolite[J]. J Chem Soc, Faraday Transactions 1, 1981, 77 (9): 2209- 2221.
doi: 10.1039/f19817702209 |
| 13 |
DAHL I M , KOLBOE S . On the reaction mechanism for hydrocarbon formation from methanol over SAPO-34:2. Isotopic labeling studies of the co-reaction of propene and methanol[J]. J Catal, 1996, 161 (1): 304- 309.
doi: 10.1006/jcat.1996.0188 |
| 14 |
MOLE T , WHITESIDE J A , SEDDON D . Aromatic co-catalysis of methanol conversion over zeolite catalysts[J]. J Catal, 1983, 82 (2): 261- 266.
doi: 10.1016/0021-9517(83)90192-6 |
| 15 |
GUISNET M , COSTA L , RIBEIRO F R . Prevention of zeolite deactivation by coking[J]. J Mol Catal A: Chem, 2009, 305 (1-2): 69- 83.
doi: 10.1016/j.molcata.2008.11.012 |
| 16 |
OLSBYE U , SVELLE S , BJØRGEN M , et al. Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity[J]. Angew Chem Int Ed, 2012, 51 (24): 5810- 5831.
doi: 10.1002/anie.201103657 |
| 17 |
STÖCKER M . Methanol-to-hydrocarbons: catalytic materials and their behavior[J]. Micropor Mesopor Mat, 1999, 29 (1-2): 3- 48.
doi: 10.1016/S1387-1811(98)00319-9 |
| 18 |
MARCUS D M , MCLACHLAN K A , WILDMAN M A , et al. Experimental evidence from H/D exchange studies for the failure of direct C-C coupling mechanisms in the methanol-to-olefin process catalyzed by HSAPO-34[J]. Angew Chem, 2006, 118 (19): 3205- 3208.
doi: 10.1002/ange.200504372 |
| 19 |
LESTHAEGHE D , VAN SPEYBROECK V , MARIN G B , et al. Understanding the failure of direct C-C Coupling in the zeolite-catalyzed methanol-to-olefin process[J]. Angew Chem, 2006, 118 (11): 1746- 1751.
doi: 10.1002/ange.200503824 |
| 20 |
DESSAU R M , LAPIERRE R B . On the mechanism of methanol conversion to hydrocarbons over HZSM-5[J]. J Catal, 1982, 78 (1): 136- 141.
doi: 10.1016/0021-9517(82)90292-5 |
| 21 |
DAHL I M , KOLBOE S . On the reaction mechanism for hydrocarbon formation from methanol over SAPO-34:I. Isotopic labeling studies of the co-reaction of ethene and methanol[J]. J Catal, 1994, 149 (2): 458- 464.
doi: 10.1006/jcat.1994.1312 |
| 22 |
WANG C , YI X F , XU J , et al. Experimental evidence on the formation of ethene through carbocations in methanol conversion over H-ZSM-5 zeolite[J]. Chem A Eur J, 2015, 21 (34): 12061- 12068.
doi: 10.1002/chem.201501355 |
| 23 | ROJO-GAMA D , SIGNORILE M , BONINO F , et al. Structure-deactivation relationships in zeolites during the methanol-to-hydrocarbons reaction: Complementary assessments of the coke content[J]. J Catal, 2017, 35133-48. |
| 24 |
MORES D , STAVITSKI E , KOX M H F , et al. Space- and time-resolved in-situ spectroscopy on the coke formation in molecular sieves: methanol-to-olefin conversion over H-ZSM-5 and H-SAPO-34[J]. Chem A Eur J, 2008, 14 (36): 11320- 11327.
doi: 10.1002/chem.200801293 |
| 25 |
GAO S S , XU S T , WEI Y X , et al. Insight into the deactivation mode of methanol-to-olefins conversion over SAPO-34:Coke, diffusion, and acidic site accessibility[J]. J Catal, 2018, 367, 306- 314.
doi: 10.1016/j.jcat.2018.09.010 |
| 26 |
WU X Q , CHEN W , XU S T , et al. Dynamic activation of C1 molecules evoked by zeolite catalysis[J]. ACS Cent Sci, 2021, 7 (4): 681- 687.
doi: 10.1021/acscentsci.1c00005 |
| 27 |
WANG S , CHEN Y Y , QIN Z F , et al. Origin and evolution of the initial hydrocarbon pool intermediates in the transition period for the conversion of methanol to olefins over H-ZSM-5 zeolite[J]. J Catal, 2019, 369, 382- 395.
doi: 10.1016/j.jcat.2018.11.018 |
| 28 |
WU X Q , XU S T , WEI Y X , et al. Evolution of C-C bond formation in the methanol-to-olefins process: From direct coupling to autocatalysis[J]. ACS Catal, 2018, 8 (8): 7356- 7361.
doi: 10.1021/acscatal.8b02385 |
| 29 |
CHOWDHURY A D , HOUBEN K , WHITING G T , et al. Initial carbon-carbon bond formation during the early stages of the methanol-to-olefin process proven by zeolite-trapped acetate and methyl acetate[J]. Angew Chem Int Ed, 2016, 55 (51): 15840- 15845.
doi: 10.1002/anie.201608643 |
| 30 |
LIU Y , MÜLLER S , BERGER D , et al. Formation mechanism of the first carbon-carbon bond and the first olefin in the methanol conversion into hydrocarbons[J]. Angew Chem Int Ed, 2016, 55 (19): 5723- 5726.
doi: 10.1002/anie.201511678 |
| 31 |
WANG W , BUCHHOLZ A , SEILER M , et al. Evidence for an initiation of the methanol-to-olefin process by reactive surface methoxy groups on acidic zeolite catalysts[J]. J Am Chem Soc, 2003, 125 (49): 15260- 15267.
doi: 10.1021/ja0304244 |
| 32 |
WANG W , HUNGER M . Reactivity of surface alkoxy species on acidic zeolite catalysts[J]. Acc Chem Res, 2008, 41 (8): 895- 904.
doi: 10.1021/ar700210f |
| 33 |
JIANG Y J , HUNGER M , WANG W . On the reactivity of surface methoxy species in acidic zeolites[J]. J Am Chem Soc, 2006, 128 (35): 11679- 11692.
doi: 10.1021/ja061018y |
| 34 |
JIANG Y J , WANG W , REDDY MARTHALA V R , et al. Effect of organic impurities on the hydrocarbon formation via the decomposition of surface methoxy groups on acidic zeolite catalysts[J]. J Catal, 2006, 238 (1): 21- 27.
doi: 10.1016/j.jcat.2005.11.029 |
| 35 |
LI J F , WEI Z H , CHEN Y Y , et al. A route to form initial hydrocarbon pool species in methanol conversion to olefins over zeolites[J]. J Catal, 2014, 317, 277- 283.
doi: 10.1016/j.jcat.2014.05.015 |
| 36 |
WANG C , CHU Y Y , XU J , et al. Extra-framework aluminum-assisted initial C-C bond formation in methanol-to-olefins conversion on zeolite H-ZSM-5[J]. Angew Chem Int Ed, 2018, 57 (32): 10197- 10201.
doi: 10.1002/anie.201805609 |
| 37 |
YANG L , YAN T T , WANG C M , et al. Role of acetaldehyde in the roadmap from initial carbon-carbon bonds to hydrocarbons during methanol conversion[J]. ACS Catal, 2019, 9 (7): 6491- 6501.
doi: 10.1021/acscatal.9b00641 |
| 38 |
WU X Q , XU S T , ZHANG W N , et al. Direct mechanism of the first carbon-carbon bond formation in the methanol-to-hydrocarbons process[J]. Angew Chem Int Ed, 2017, 56 (31): 9039- 9043.
doi: 10.1002/anie.201703902 |
| 39 |
SUN T T , CHEN W , XU S T , et al. The first carbon-carbon bond formation mechanism in methanol-to-hydrocarbons process over chabazite zeolite[J]. Chem, 2021, 7 (9): 2415- 2428.
doi: 10.1016/j.chempr.2021.05.023 |
| 40 |
YARULINA I , CHOWDHURY A D , MEIRER F , et al. Recent trends and fundamental insights in the methanol-to-hydrocarbons process[J]. Nat Catal, 2018, 1 (6): 398- 411.
doi: 10.1038/s41929-018-0078-5 |
| 41 |
HAW J F , SONG W G , MARCUS D M , et al. The mechanism of methanol to hydrocarbon catalysis[J]. Acc Chem Res, 2003, 36 (5): 317- 326.
doi: 10.1021/ar020006o |
| 42 |
ILIAS S , BHAN A . Mechanism of the catalytic conversion of methanol to hydrocarbons[J]. ACS Catal, 2013, 3 (1): 18- 31.
doi: 10.1021/cs3006583 |
| 43 |
BJØRGEN M , SVELLE S , JOENSEN F , et al. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5:On the origin of the olefinic species[J]. J Catal, 2007, 249 (2): 195- 207.
doi: 10.1016/j.jcat.2007.04.006 |
| 44 |
ARSTAD B , NICHOLAS J B , HAW J F . Theoretical study of the methylbenzene side-chain hydrocarbon pool mechanism in methanol to olefin catalysis[J]. J Am Chem Soc, 2004, 126 (9): 2991- 3001.
doi: 10.1021/ja035923j |
| 45 |
SONG W G , HAW J F , NICHOLAS J B , et al. Methylbenzenes are the organic reaction centers for methanol-to-olefin catalysis on HSAPO-34[J]. J Am Chem Soc, 2000, 122 (43): 10726- 10727.
doi: 10.1021/ja002195g |
| 46 |
MCCANN D M , LESTHAEGHE D , KLETNIEKS P W , et al. A complete catalytic cycle for supramolecular methanol-to-olefins conversion by linking theory with experiment[J]. Angew Chem Int Ed, 2008, 47 (28): 5179- 5182.
doi: 10.1002/anie.200705453 |
| 47 |
ZHANG W N , ZHANG M Z , XU S T , et al. Methylcyclopentenyl cations linking initial stage and highly efficient stage in methanol-to-hydrocarbon process[J]. ACS Catal, 2020, 10 (8): 4510- 4516.
doi: 10.1021/acscatal.0c00799 |
| 48 |
XIAO D , XU S T , BROWNBILL N J , et al. Fast detection and structural identification of carbocations on zeolites by dynamic nuclear polarization enhanced solid-state NMR[J]. Chem Sci, 2018, 9 (43): 8184- 8193.
doi: 10.1039/C8SC03848A |
| 49 |
ZHANG M Z , XU S T , LI J Z , et al. Methanol to hydrocarbons reaction over Hβ zeolites studied by high resolution solid-state NMR spectroscopy: Carbenium ions formation and reaction mechanism[J]. J Catal, 2016, 335, 47- 57.
doi: 10.1016/j.jcat.2015.12.007 |
| 50 |
OLSBYE U , SVELLE S , LILLERUD K P , et al. The formation and degradation of active species during methanol conversion over protonated zeotype catalysts[J]. Chem Soc Rev, 2015, 44 (20): 7155- 7176.
doi: 10.1039/C5CS00304K |
| 51 |
SONG W , MARCUS D M , FU H , et al. An oft-studied reaction that may never have been: Direct catalytic conversion of methanol or dimethyl ether to hydrocarbons on the solid acids HZSM-5 or HSAPO-34[J]. J Am Chem Soc, 2002, 124 (15): 3844- 3845.
doi: 10.1021/ja016499u |
| 52 |
MINOVA I B , MATAM S K , GREENAWAY A , et al. Elementary steps in the formation of hydrocarbons from surface methoxy groups in HZSM-5 seen by synchrotron infrared microspectroscopy[J]. ACS Catal, 2019, 9 (7): 6564- 6570.
doi: 10.1021/acscatal.9b01820 |
| 53 |
HUNGER M . In situ NMR spectroscopy in heterogeneous catalysis[J]. Catal Today, 2004, 97 (1): 3- 12.
doi: 10.1016/j.cattod.2004.03.061 |
| 54 |
HAW J F , GOGUEN P W , XU T , et al. In situ NMR investigations of heterogeneous catalysis with samples prepared under standard reaction conditions[J]. Angew Chem Int Ed, 1998, 37 (7): 948- 949.
doi: 10.1002/(SICI)1521-3773(19980420)37:7<948::AID-ANIE948>3.0.CO;2-L |
| 55 |
LI J Z , WEI Y X , CHEN J , et al. Observation of heptamethylbenzenium cation over SAPO-type molecular sieve DNL-6 under real MTO conversion conditions[J]. J Am Chem Soc, 2012, 134 (2): 836- 839.
doi: 10.1021/ja209950x |
| 56 |
WANG J B , WEI Y X , LI J Z , et al. Direct observation of methylcyclopentenyl cations (MCP+) and olefin generation in methanol conversion over TON zeolite[J]. Catal Sci Technol, 2016, 6 (1): 89- 97.
doi: 10.1039/C5CY01420D |
| 57 |
CHEN J R , LI J Z , YUAN C Y , et al. Elucidating the olefin formation mechanism in the methanol to olefin reaction over AlPO-18 and SAPO-18[J]. Catal Sci Technol, 2014, 4 (9): 3268- 3277.
doi: 10.1039/C4CY00551A |
| 58 |
LI J Z , WEI Y X , CHEN J R , et al. Cavity controls the selectivity: Insights of confinement effects on MTO reaction[J]. ACS Catal, 2015, 5 (2): 661- 665.
doi: 10.1021/cs501669k |
| 59 |
ZHANG M , XU S T , WEI Y X , et al. Methanol conversion on ZSM-22, ZSM-35 and ZSM-5 zeolites: effects of 10-membered ring zeolite structures on methylcyclopentenyl cations and dual cycle mechanism[J]. RSC Adv, 2016, 6 (98): 95855- 95864.
doi: 10.1039/C6RA08884H |
| 60 |
ZHANG W N , CHEN J R , XU S T , et al. Methanol to olefins reaction over cavity-type zeolite: Cavity controls the critical intermediates and product selectivity[J]. ACS Catal, 2018, 8 (12): 10950- 10963.
doi: 10.1021/acscatal.8b02164 |
| 61 | ZHANG W N , XU S T , ZHI Y C , et al. Methylcyclopentenyl cation mediated reaction route in methanol-to-olefins reaction over H-RUB-50 with small cavity[J]. J Energy Chem, 2020, 4525- 30. |
| 62 |
XIAO D , XU S T , HAN X W , et al. Direct structural identification of carbenium ions and investigation of host-guest interaction in the methanol to olefins reaction obtained by multinuclear NMR correlations[J]. Chem Sci, 2017, 8 (12): 8309- 8314.
doi: 10.1039/C7SC03657D |
| 63 |
NI Q Z , DAVISO E , CAN T V , et al. High frequency dynamic nuclear polarization[J]. Acc Chem Res, 2013, 46 (9): 1933- 1941.
doi: 10.1021/ar300348n |
| 64 |
LESAGE A , LELLI M , GAJAN D , et al. Surface enhanced NMR spectroscopy by dynamic nuclear polarization[J]. J Am Chem Soc, 2010, 132 (44): 15459- 15461.
doi: 10.1021/ja104771z |
| 65 |
XIAO J R , WEI J . Diffusion mechanism of hydrocarbons in zeolites-Ⅱ. Analysis of experimental observations[J]. Chem Eng Sci, 1992, 47 (5): 1143- 1159.
doi: 10.1016/0009-2509(92)80237-7 |
| 66 |
SMIT B , MAESEN T L M . Towards a molecular understanding of shape selectivity[J]. Nature, 2008, 451 (7179): 671- 678.
doi: 10.1038/nature06552 |
| 67 | ZHOU J , FAN W , WANG Y D , et al. The essential mass transfer step in hierarchical/nano zeolite: surface diffusion[J]. Natl Sci Rev, 2019, 7 (11): 1630- 1632. |
| 68 | GAO S S , LIU Z Q , XU S T , et al. Cavity-controlled diffusion in 8-membered ring molecular sieve catalysts for shape selective strategy[J]. J Catal, 2019, 37751-62. |
| 69 |
ZENG S , XU S T , GAO S S , et al. Differentiating diffusivity in different channels of ZSM-5 zeolite by pulsed field gradient (PFG) NMR[J]. ChemCatChem, 2020, 12 (2): 463- 468.
doi: 10.1002/cctc.201901689 |
| 70 |
HAN J F , LIU Z Q , LI H , et al. Simultaneous evaluation of reaction and diffusion over molecular sieves for shape-selective catalysis[J]. ACS Catal, 2020, 10 (15): 8727- 8735.
doi: 10.1021/acscatal.0c02054 |
| 71 |
PENG S C , GAO M B , LI H , et al. Control of surface barriers in mass transfer to modulate methanol-to-olefins reaction over SAPO-34 zeolites[J]. Angew Chem Int Ed, 2020, 59 (49): 21945- 21948.
doi: 10.1002/anie.202009230 |
| 72 |
DAI W L , SCHEIBE M , LI L D , et al. Effect of the methanol-to-olefin conversion on the PFG NMR self-diffusivities of ethane and ethene in large-crystalline SAPO-34[J]. J Phys Chem C, 2012, 116 (3): 2469- 2476.
doi: 10.1021/jp208815g |
| 73 |
CNUDDE P , REDEKOP E A , DAI W , et al. Experimental and theoretical evidence for the promotional effect of acid sites on the diffusion of alkenes through small-pore zeolites[J]. Angew Chem Int Ed, 2021, 60 (18): 10016- 10022.
doi: 10.1002/anie.202017025 |
| 74 |
KÄRGER J . Transport Phenomena in Nanoporous Materials[J]. ChemPhysChem, 2015, 16 (1): 24- 51.
doi: 10.1002/cphc.201402340 |
| 75 |
KÄRGER J , VALIULLIN R . Mass transfer in mesoporous materials: the benefit of microscopic diffusion measurement[J]. Chem Soc Rev, 2013, 42 (9): 4172- 4197.
doi: 10.1039/c3cs35326e |
| 76 |
SMIT B , MAESEN T L M . Molecular simulations of zeolites: Adsorption, diffusion, and shape selectivity[J]. Chem Rev, 2008, 108 (10): 4125- 4184.
doi: 10.1021/cr8002642 |
| 77 |
BUURMANS I L C , WECKHUYSEN B M . Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy[J]. Nat Chem, 2012, 4 (11): 873- 886.
doi: 10.1038/nchem.1478 |
| 78 | LOSCH P , PINAR A B , WILLINGER M G , et al. H-ZSM-5 zeolite model crystals: Structure-diffusion-activity relationship in methanol-to-olefins catalysis[J]. J Catal, 2017, 34511-23. |
| 79 |
SHEN Y F , LE T T , FU D L , et al. Deconvoluting the competing effects of zeolite framework topology and diffusion path length on methanol to hydrocarbons reaction[J]. ACS Catal, 2018, 8 (12): 11042- 11053.
doi: 10.1021/acscatal.8b02274 |
| 80 |
SPRINGUEL-HUET M A , BONARDET J L , GéDéON A , et al. 129Xe NMR overview of xenon physisorbed in porous solids[J]. Magn Reson Chem, 1999, 37 (13): S1- S13.
doi: 10.1002/(SICI)1097-458X(199912)37:13<S1::AID-MRC578>3.0.CO;2-X |
| 81 | WEILAND E , SPRINGUEL-HUET M A , NOSSOV A , et al. 129Xenon NMR: Review of recent insights into porous materials[J]. Microporous Mesoporous Mater, 2016, 225 (1): 41- 65. |
| 82 |
GAO S S , XU S T , WEI Y X , et al. Direct probing of heterogeneity for adsorption and diffusion within a SAPO-34 crystal[J]. Chem Commun, 2019, 55 (72): 10693- 10696.
doi: 10.1039/C9CC05322K |
| 83 |
GAO S S , YUAN J M , LIU Z Q , et al. Correlating the adsorption preference and mass transfer of xenon in RHO-type molecular sieves[J]. J Phys Chem C, 2021, 125 (12): 6832- 6838.
doi: 10.1021/acs.jpcc.0c10813 |
| 84 |
LOU C Y , ZHANG W N , MA C , et al. Revealing the specific spatial confinement in 8-membered ring cage-type molecular sieves via solid-state NMR and theoretical calculations[J]. ChemCatChem, 2021, 13 (5): 1299- 1305.
doi: 10.1002/cctc.202001682 |
| 85 |
ANDERSON M W , KLINOWSKI J . Direct observation of shape selectivity in zeolite ZSM-5 by magic-angle-spinning NMR[J]. Nature, 1989, 339 (6221): 200- 203.
doi: 10.1038/339200a0 |
| 86 |
WEI Y X , YUAN C Y , LI J Z , et al. Coke formation and carbon atom economy of methanol-to-olefins reaction[J]. ChemSusChem, 2012, 5 (5): 906- 912.
doi: 10.1002/cssc.201100528 |
| [1] | Rui QIN,Chao WANG,Qiang WANG,Min HU,Jin-lin LI,Jun XU,Feng DENG. Formation and Reactivity of Surface Methoxy Species in Methanol Conversion over SSZ-13 Zeolite [J]. Chinese Journal of Magnetic Resonance, 2022, 39(4): 439-447. |
| [2] | Yao XIAO,Chang-jiu XIA,Xian-feng YI,Feng-qing LIU,Shang-bin LIU,An-min ZHENG. Progress in the Studies on Sn-Zeolites by Solid-State Nuclear Magnetic Resonance [J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 571-584. |
| [3] | YUAN Chen-lu, GUO Qian-ni, CHEN Shi-zhen, ZHOU Xin. A Novel Molecular Cage for Hyperpolarized 129Xe Based on Cucurbit[6]uril Nanoparticles [J]. Chinese Journal of Magnetic Resonance, 2019, 36(4): 472-480. |
| [4] | LI Bo-jie,XU Jun*,WANG Qiang,WANG Xiu-mei,QI Guo-dong,DENG Feng*. Carbonylation of Methanol on Cu-H-MOR Zeolites: Insights from Solid-State NMR Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2014, 31(3): 331-340. |
| [5] | LI Fang1,3;ZHAO Ying-sheng2;LEI Ai-wen2;LIU Mai-li1*. Palladium Catalyzed Csp-Csp3 Bond Formation Reaction Studied by 13C NMR [J]. Chinese Journal of Magnetic Resonance, 2008, 25(4): 461-469. |
| [6] |
YANG Ying1; YAN Bao-zhen1; NIE Zhou2; WANG Mei1; TIAN Qiu2; LIU Yang2* . The Mechanism of a Reaction between α-Tocopherol and DPPH·Studied by NMR and ESR Spectroscopy [J]. Chinese Journal of Magnetic Resonance, 2008, 25(3): 331-336. |
| [7] | YE Jian-Liang, ZHENG Fa-Kun, CHEN Zhong, XIE Hong-Chen, CHEN Zhi-Wei, HUANG Pei-Qiang. 51V NMR STUDIES ON THE REACTION OF [VS4Cun](n=3-6) CLUSTERS [J]. Chinese Journal of Magnetic Resonance, 2001, 18(4): 315-320. |
| [8] | YANG Xiuwei. COMPLETE ASSIGNMENT OF 1H AND 13C NMR CHEMICAL SHIFTS OF 20(R)-GINSENOSIDE Rg2 AND 20(S)-GINSENOSIDE Rg2 [J]. Chinese Journal of Magnetic Resonance, 2000, 17(1): 9-15. |
| [9] | ZHANG Hu, YANG Xiuwei, CHUI Yuxin. COMPLETE ASSIGNMENT OF 1H AND 13C NMR CHEMICAL SHIFTS OF EVODIAMINE,RUTAECARPINE AND DEHYDROEVODIAMINE [J]. Chinese Journal of Magnetic Resonance, 1999, 16(6): 563-568. |
| [10] | Cao Rong, Wu Daxu, Xie Xiulan, Hong Maochun, Jiang Feilong, Liu Hanqin. STUDIES ON THE NUCLEAR MA GNETIC RESONANCE OF MOLYBDENUM (TUNGSTEN)-COPPER-SULPHUR-DIALKYLDI-THIOCARBAMATE CLUSTER COMPOUNDS [J]. Chinese Journal of Magnetic Resonance, 1995, 12(1): 13-21. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||