引言
CEST成像脉冲序列通常包括两个部分:饱和模块与采集模块. 饱和模块的作用是产生CEST对比度,而采集模块则是获取具有CEST对比度的MRI图像[11]. 因此,采集模块的设计会极大程度上影响CEST图像的质量以及CEST对比度的准确性. 快速自旋回波序列(Turbo Spin Echo,TSE)[12]、梯度回波序列(Gradient Echo,GRE)[11,13⇓-15]和回波平面成像技术(Echo Planar Imaging,EPI)[16,17]都可以作为CEST序列的采集模块. TSE序列可以获得高信噪比的图像,但是扫描时间较长,而且采集过程中CEST对比度的持续衰减会降低扫描结果的准确性. 采用EPI方法的序列成像速度很快,但其图像信噪比很低,并且存在磁化率伪影[18]、N/2伪影[19]等问题. GRE序列作为一种快速MRI序列,其成像速度比TSE序列更快,图像信噪比比EPI方法更高,常作为CEST实验的采集序列. 而作为GRE序列的一种,快速小角度激发(Fast Low Angle Shot,FLASH)序列加入了扰相梯度,可以消除快速采集过程中残余横向磁化矢量的影响[20].
然而,在分段饱和采集过程中,由于磁化矢量的演化历程被重复中断,这在K空间填充过程中造成了磁化矢量演化的不连续性,不同的K空间填充策略会在相位编码方向上引起磁化矢量不同程度的幅度调制. 特别在CEST的饱和过程后,由于脂肪比水的T1弛豫时间短,其磁化矢量强度会快速恢复,这会在K空间相位编码方向上引发更为显著的幅度调制效应,最终可能会在CEST图像中产生更明显的鬼影状伪影. 针对该问题,本文通过理论仿真和人体实验,探究了FLASH序列中不同K空间填充策略对颅脑APT图像质量以及APT非对称分析定量的影响,证实了K空间分段重排的中心优先填充技术,可有效消除皮下脂肪导致的伪影,并提高APT非对称分析定量的准确性.
1 材料与方法
1.1 仿真实验
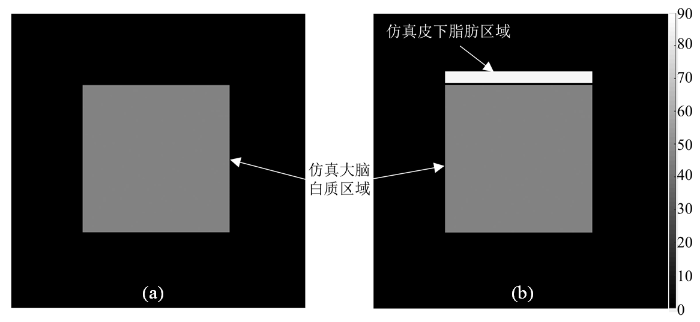
仿真实验使用MATLAB R2019b编程实现. 为了便于观察与计算,仿真实验在128×128像素的图像上选取图像中心64×64像素的区域模仿横断面大脑组织,相关仿真参数基于大脑白质. 在图像内侧上方选取64×5像素的区域模仿头部皮下脂肪,具体位置关系如图1中所示.
图1
图1
仿真图像. (a)不考虑皮下脂肪的仿真图像;(b)考虑皮下脂肪的仿真图像. 图像中央方形区域仿真大脑白质. 图内侧上方条形区域仿真皮下脂肪
Fig. 1
Simulated images. (a) Simulated image without considering scalp fat; (b) Simulated image considering scalp fat. The square area in the center simulates brain white matter. The bar-shaped area on the upper inside simulates scalp fat
根据FLASH序列信号强度公式生成各区域的理论磁共振信号强度图,将理论磁共振信号强度图通过傅里叶变换得到理论上的原始K空间数据. 通过对原始K空间在相位编码方向进行信号演化,得到不同K空间排列情况对应的K空间数据,再将其进行傅里叶逆变换到图像空间,以获得不同K空间演化情况下的磁共振信号强度图. 为了简化模型,仿真实验中忽略了脂肪和水的共振频率差导致的频率编码方向图像偏移.
理论上MRI信号强度可通过将对应的组织生理参数(
其中,
表1 仿真实验使用的组织生理参数
Table 1
| 质子密度 | T1/ms | T2*/ms | 饱和幅度/% | |
|---|---|---|---|---|
| 皮下脂肪(δ -3.5) | 91.1% | 382 | 68 | 80 |
| 大脑白质(δ 3.5) | 68.6% | 1087 | 56 | 10 |
本文对五种不同的K空间填充策略进行了仿真,分别为:(A)单次饱和与顺序采集;(B)单次饱和与中心优先采集;(C)分段饱和与顺序采集;(D)分段饱和与中心优先采集;(E)分段饱和与重排优化的中心优先采集. 分段饱和将整个采集过程分为四段,每段K空间采集完成后设置一定的恢复时间,使得纵向磁化矢量恢复至最大值,施加饱和模块后再采集下一段K空间. 表2展示了以128条K空间线为例的五种K空间填充策略的采集顺序. 在此,我们使用数字的大小顺序来代表采集顺序,其中#号之后的数字表示的是分段数,例如#1-1代表第1段K空间的第1次采集.
表2 五种K空间填充策略下的不同K空间采集顺序
Table 2
| K空间 相位编码顺序 | 单次饱和与 顺序采集 | 单次饱和与 中心优先采集 | 分段饱和与 顺序采集 | 分段饱和与 中心优先采集 | 分段饱和与 重排中心优先采集 |
|---|---|---|---|---|---|
| 1 | 1 | 127 | #1-1 | #4-31 | #3-32 |
| 2 | 2 | 125 | #1-2 | #4-29 | #1-32 |
| : | : | : | : | : | : |
| 16 | 16 | 97 | #1-16 | #4-1 | #1-25 |
| 17 | 17 | 95 | #1-17 | #3-31 | #3-24 |
| : | : | : | : | : | : |
| 32 | 32 | 65 | #1-32 | #3-1 | #1-17 |
| 33 | 33 | 63 | #2-1 | #2-31 | #3-16 |
| : | : | : | : | : | : |
| 48 | 48 | 33 | #2-16 | #2-1 | #1-9 |
| 49 | 49 | 31 | #2-17 | #1-31 | #3-8 |
| : | : | : | : | : | : |
| 63 | 63 | 3 | #2-31 | #1-3 | #3-1 |
| 64 | 64 | 1 | #2-32 | #1-1 | #1-1 |
| 65 | 65 | 2 | #3-1 | #1-2 | #2-1 |
| 66 | 66 | 4 | #3-2 | #1-4 | #4-1 |
| : | : | : | : | : | : |
| 80 | 80 | 32 | #3-16 | #1-32 | #4-8 |
| 81 | 81 | 34 | #3-17 | #2-2 | #2-9 |
| : | : | : | : | : | : |
| 96 | 96 | 64 | #3-32 | #2-32 | #4-16 |
| 97 | 97 | 66 | #4-1 | #3-2 | #2-17 |
| : | : | : | : | : | : |
| 112 | 112 | 96 | #4-16 | #3-32 | #4-24 |
| 113 | 113 | 98 | #4-17 | #4-2 | #2-25 |
| : | : | : | : | : | : |
| 127 | 127 | 126 | #4-31 | #4-30 | #2-32 |
| 128 | 128 | 128 | #4-32 | #4-32 | #4-32 |
注:分段饱和时,#i-j中i代表段编号,j代表段内采集编号. 分段饱和与重排中心优先采集方法的K空间填充顺序为:K空间上半区域由奇数段交替填充,K空间下半区域由偶数段交替填充.
在不考虑主磁场不均匀性带来的相位离散影响时,K空间演化过程由FLASH序列连续采集的纵向磁化矢量公式获得[28]:
其中,
仿真实验中模拟真实的CEST数据后处理流程,使用非对称分析获取非对称性磁化转移率(Magnetization Transfer Ratio Asymmetry,MTRasym)用以表征APT对比度:
其中,
在仿真实验中,通过模拟不同采集方法情况下CEST信号采集与理论饱和程度的差异,用以反映不同K空间分段和排列方法对APT图像定量结果的影响. 使用ITK-SNAP图像处理软件[29](
1.2 人体实验
招募了5例健康志愿者被试(其中男性2名,女性3名,年龄范围为19~23岁)进行人体实验,所有受试者均无MRI扫描禁忌症,也无神经学、心血管或其他严重躯体疾病史. 本研究得到了华东师范大学人体试验伦理委员会的批准(批准文号:HR 319-2022),受试者均自愿参加并签署知情同意书.
人体实验数据在西门子Prisma Fit 3 T仪器上采集,使用64通道头颈联合线圈进行信号接收. 本研究中所用序列在西门子面向应用程序的集成开发环境(Integrated Development Environment for Applications,IDEA)平台上自主开发完成,在常规的二维FLASH序列的基础上实现了五种不同的单段或分段CEST饱和策略及K空间填充顺序. 扫描参数如下:单层采集,层面方向为横断面,层厚为6 mm,观察野为220×220 mm2,采集矩阵为64×64,由西门子图像重建系统插值至128×128,相位编码方向为前后,TR为8.35 ms,TE为4.5 ms,FA为14˚,采集带宽为320 Hz/pixel. CEST饱和模块由10个持续时间为200 ms、间隔时间为5 ms的高斯型射频脉冲组成,饱和功率为2 μT. 实验采集以水频率为中心、频率偏移为δ -10至δ 10共41张图像,频率间隔为δ 0.5,最后再采集一幅不施加饱和射频脉冲的参考图像,采集顺序由Z谱两端交替向水频率中心靠近,并在每一个射频饱和频率采集完成后增加4 s的等待时间以保证纵向磁化矢量得以恢复. 五种K空间填充策略的扫描序列除K空间填充策略外,其余扫描参数均保持一致,其中分段饱和采集的序列扫描时间为10 min 15 s,单段饱和采集的序列扫描时间为4 min 54 s.
2 结果与讨论
2.1 仿真实验结果
图2展示了饱和频率为δ -3.5时脂肪信号K空间幅度调制曲线及对应的图像空间点扩散函数. 从图2中可以看到,在分段饱和与顺序采集、分段饱和与中心优先采集的情况下,由于K空间演化经历了分段的幅度调制,其对应在图像空间的点扩散函数由单峰变为多峰[图2(h), (i)],这说明了图像空间中脂肪信号会向周围空间扩散,造成伪影,而其他三种方法的点扩散函数仍为单峰,说明脂肪信号对附近图像造成的影响较小.
图2
图2
五种不同K空间填充策略下饱和脂肪信号在采集过程的幅度调制曲线(第一行)及其对应的图像空间点扩散函数(第二行). (a, f)单次饱和与顺序采集;(b, g)单次饱和与中心优先采集;(c, h)分段饱和与顺序采集;(d, i)分段饱和与中心优先采集;(e, j)分段饱和与重排中心优先采集
Fig. 2
Amplitude modulation curves after fat signal saturation for five different K-space filling strategies (the first row) and the corresponding point spread functions in image space (the second row). (a, f) single saturation with sequential acquisition; (b, g) single saturation with center-out acquisition; (c, h) multiple saturations with sequential acquisition; (d, i) multiple saturations with center-out acquisition; (e, j) multiple saturations with reordered center-out acquisition
图3
图3
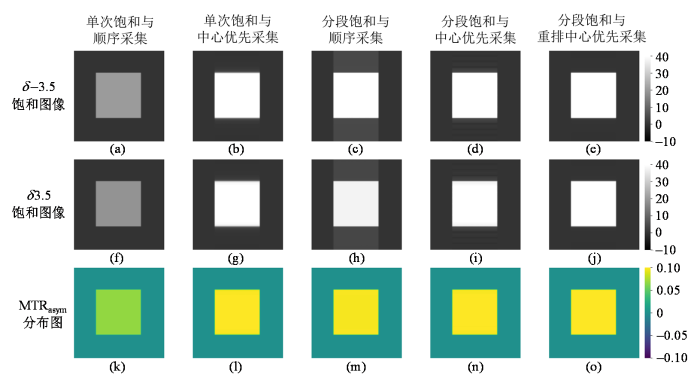
不考虑皮下脂肪时仿真得到的δ -3.5处饱和图像(第一行)、δ 3.5处饱和图像(第二行)和MTRasym分布图(第三行). (a, f, k)单次饱和与顺序采集方法;(b, g, l)单次饱和与中心优先采集方法;(c, h, m)分段饱和与顺序采集方法;(d, i, n)分段饱和与中心优先采集方法;(e, j, o)分段饱和与重排中心优先采集方法
Fig. 3
Simulated saturation images with saturation frequency at δ -3.5 (the first row) and δ 3.5 (the second row), and MTRasym maps (the third row), obtained without considering scalp fat. (a, f, k) single saturation with sequential acquisition; (b, g, l) single saturation with center-out acquisition; (c, h, m) segmental saturation with sequential acquisition; (d, i, n) segmental saturation with center-out acquisition; and (e, j, o) segmental saturation with reordered center-out acquisition
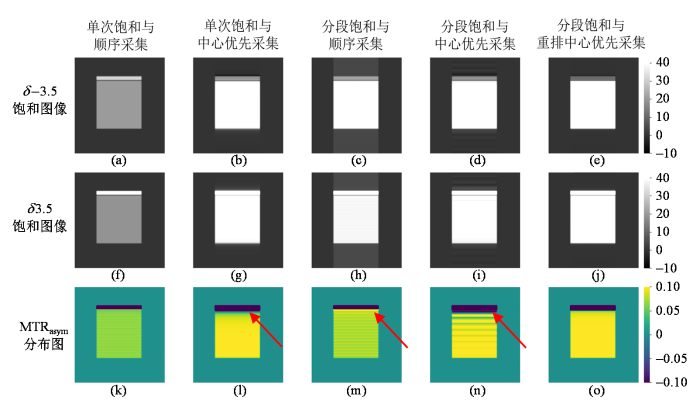
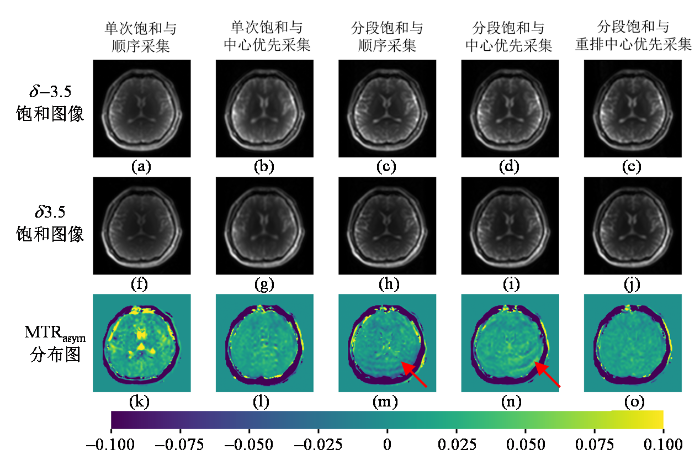
图4为考虑皮下脂肪存在时的仿真实验的结果,分别展示了仿真中模拟采用五种K空间填充策略获取的δ -3.5、δ 3.5频率偏移处的饱和图像以及对应的MTRasym定量图,其中皮下脂肪的饱和幅度理论值为80%,大脑组织的饱和幅度理论值为10%,相位编码方向为前后方向. 考虑皮下脂肪后,不同的K空间填充策略采集仿真得到的图像伪影出现相当明显的差异. 单次饱和方法得到的饱和图像中白质区域信号均匀[图4(a)、(b)、(f)和(g)],但是单次饱和与顺序采集方法得到的MTRasym图中有明显的细条纹状伪影,而且MTRasym明显比理论值偏低[图4(k)];而单次饱和与中心优先采集方法得到的MTRasym定量图上虽然没有条纹状伪影,但其靠近脂肪的区域信号强度明显下降[图4(l)上红色箭头所示]. 分段饱和与顺序采集方法、分段饱和与中心优先采集方法这两种策略得到的饱和图像上有明显的条纹状伪影[图4(c)、(d)、(h)和(i)],进而在MTRasym定量图上靠近脂肪区域的伪影更加严重[图4(m)和(n)上红色箭头所示]. 分段饱和与重排中心优先采集方法得到的MTRasym定量图最为均匀,受脂肪伪影影响最小[图4(o)].
图4
图4
考虑皮下脂肪时仿真得到的δ -3.5处饱和图像(第一行)、δ 3.5处饱和图像(第二行)和MTRasym分布图(第三行). (a, f, k)单次饱和与顺序采集方法;(b, g, l)单次饱和与中心优先采集方法;(c, h, m)分段饱和与顺序采集方法;(d, i, n)分段饱和与中心优先采集方法;(e, j, o)分段饱和与重排中心优先采集方法
Fig. 4
Simulated saturation images with saturation frequency at δ -3.5 (the first row) and δ 3.5 (the second row), and MTRasym maps (the third row), obtained when considering scalp fat. (a, f, k) single saturation with sequential acquisition; (b, g, l) single saturation with center-out acquisition; (c, h, m) segmental saturation with sequential acquisition; (d, i, n) segmental saturation with center-out acquisition; and (e, j, o) segmental saturation with reordered center-out acquisition
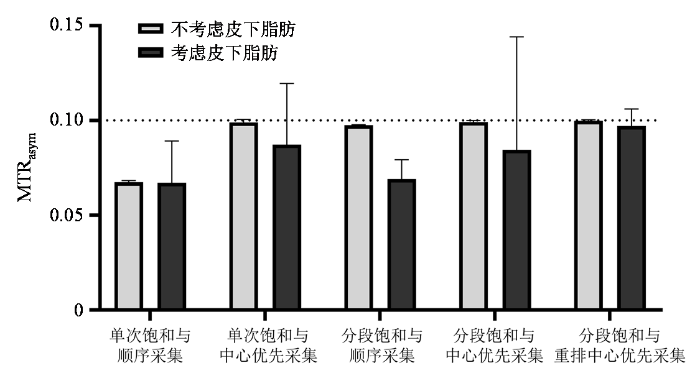
图5为仿真实验的定量统计结果,分别统计了有/无皮下脂肪情况下仿真大脑白质区域MTRasym的平均值和标准差. 可以看到在不考虑皮下脂肪时,除单次饱和与顺序采集方法外,其余K空间填充策略得到的MTRasym均接近理论值. 而考虑皮下脂肪影响后,只有分段饱和与重排中心优先采集方法仿真得到的MTRasym平均值接近于理论值,且标准差较小;而其他K空间采集策略仿真得到的MTRasym平均值均低于理论值,而且标准差较大,表示图像均匀性变差. 值得注意的是,考虑皮下脂肪的情况下分段饱和与顺序采集得到的MTRasym平均值只比单次饱和结果略高,与不考虑皮下脂肪的情况差异较大,表明分段饱和采集的脂肪伪影会显著影响MTRasym结果.
图5
图5
仿真实验的MTRasym定量统计结果
Fig. 5
Quantitative statistical results of MTRasym from simulation experiments
2.2 人体实验结果
图6
图6
一例正常志愿者颅脑扫描得到的δ -3.5处饱和图像(第一行)、δ 3.5处饱和图像(第二行)和MTRasym分布图(第三行). (a, f, k)为单次饱和与顺序采集方法;(b, g, l)为单次饱和与中心优先采集方法;(c, h, m)为分段饱和与顺序采集方法;(d, i, n)为分段饱和与中心优先采集方法;(e, j, o)为分段饱和与重排中心优先采集方法
Fig. 6
Brain saturation images with saturation frequency at δ -3.5 (the first row) and δ 3.5 (the second row), and MTRasym maps (the third row), obtained from a healthy volunteer. (a, f, k) single saturation with sequential acquisition; (b, g, l) single saturation with center-out acquisition; (c, h, m) segmental saturation with sequential acquisition; (d, i, n) segmental saturation with center-out acquisition; and (e, j, o) segmental saturation with reordered center-out acquisition
2.3 讨论
由于脂肪的T1弛豫时间远短于水和大脑灰白质,因此在CEST快速采集序列中脂肪对图像的影响很大,通常需要引入脂肪抑制技术[33]. 由于CEST序列加入了饱和模块,在特定饱和频率后采集的信号通常伴随着对应的饱和信号恢复过程,而脂肪的T1弛豫时间很短,使得实验采集的脂肪信号变化明显. 仿真实验显示,在单次饱和采集的K空间填充策略中,脂肪信号幅度调制对应的点扩散函数为单峰,因此饱和图像中信号均匀,但由于脂肪信号饱和与不饱和的情况下其点扩散函数是不一样的,因此在MTRasym图像上会产生明显的伪影[图4(k)和(l)]. 而在分段饱和采集方法中,由于填充K空间的脂肪信号受分段策略影响,脂肪信号在段与段之间会出现不连续的变化. 而当K空间数据出现不连续的变化时,对应的图像空间中便会出现明显的点扩散伪影. 仿真实验中,对脂肪信号施加的不同K空间幅度调制方式对应了不同的图像空间点扩散函数,在分段饱和且不重排的采集方法中,图像点扩散函数均表现为多峰,会导致脂肪信号扩散到大脑区域产生伪影,这与仿真的图像结果相对应. 在仿真结果中分段饱和的顺序采集与中心优先采集得到的饱和图像均在相位编码方向上表现出规律的条纹状伪影,且靠近脂肪区域受影响更严重,而这一现象在单次饱和采集的顺序采集与中心优先采集情况均不存在. 因此,在分段饱和与中心优先采集方法的基础上,我们的重排采集方案将K空间分段和排列顺序重新设计,使得K空间幅度调制函数变得连续,获得与单次饱和的情况类似的单峰点扩散函数,消除了脂肪信号幅度调制产生的图像伪影. 另外,由于伪影更少,分段饱和与重排中心优先采集方法得到的MTRasym定量结果更加均匀,且APT效应最接近仿真设定的理论值.
CEST的定量研究对于临床应用来说是不可或缺的[34⇓⇓⇓⇓⇓-40],其中CEST采集过程的序列方法优化不仅对图像质量起着至关重要的作用,也是CEST数据后处理方法应用的基础. 在考虑皮下脂肪的仿真实验中,单次饱和与分段饱和的顺序填充K空间得到的MTRasym平均值比较接近,其原因可能是仿真K空间相位编码次数比较少,但是MTRasym图像上脂肪伪影表现差异较大. 在人体APT实验可以看到,单次饱和与顺序采集方法的APT对比度最不均匀[图6(k)];单次饱和与中心优先采集方法没有明显的伪影影响,但其图像均匀度略差[图6(i)];分段饱和与顺序采集的脑内处理结果中靠近脑后皮下脂肪的区域出现了明显图像伪影[图6(m)];分段饱和与中心优先采集方法的脂肪伪影更加严重[图6(n)];而分段饱和与重排中心优先采集方法很好地抑制了脂肪信号幅度调制导致的APT图像伪影,图像均匀度最佳[图6(o)],这符合文献中2 μT饱和时的健康被试的APT结果表现[7,41],其脑组织灰白质非对称分析数值结果有较高的一致性. 因此,在APT非对称分析的相关应用研究中,最好采用分段饱和与重排中心优先采集方法,以提高APT的图像质量和定量的准确性.
本文的研究还存在进一步改进的空间. 首先,本文仅分析了K空间采集策略对APT非对称分析结果的影响,后续可以进一步研究K空间采集策略对洛伦兹拟合[39]、多池Bloch方程拟合[36]、深度学习[42⇓⇓-45]等算法结果的影响. 其次,对于CEST序列的扫描方案,分段饱和方法的成像时间显著长于单次饱和方法,这会限制分段饱和CEST序列在临床上的应用,在只采用非对称方法分析APT效应时,可以减少施加饱和频率偏移的范围以缩短扫描时间. 此外,对于K空间的填充顺序,本研究仅考虑了顺序填充与中心向外填充两种情况,而螺旋采集[11,46]、间隔填充[47]、K空间欠采[48]等情况未进行讨论,未来的研究可以进一步优化K空间填充策略,提高图像采集的速度和质量. 再者,本文仅分析了二维图像的K空间演化关系,由于采集K空间数据少,分段饱和采集的对比度优势并没有体现,而对于更复杂的三维CEST序列K空间填充的分段采集方案需要进一步研究. 最后,除了脂肪信号外,中继核奥式增强效应[49](δ -3.5附近)和磁化转移效应非对称性[50],对非对称分析中APT信号也会产生影响,后续也需要进一步研究.
3 结论
本研究提出了一种应用于基于FLASH的CEST成像序列的分段饱和与K空间重排采集方案,可降低分段饱和采集过程中K空间的不连续性.该方法成功抑制了皮下脂肪对颅脑APT处理结果造成的影响,提高颅脑APT非对称分析定量的准确性和稳定性,对于CEST的定量研究和临床应用可发挥积极作用.
利益冲突
无
参考文献
A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST)
[J].
DOI:10.1006/jmre.1999.1956
PMID:10698648
[本文引用: 1]

It has been previously shown that intrinsic metabolites can be imaged based on their water proton exchange rates using saturation transfer techniques. The goal of this study was to identify an appropriate chemical exchange site that could be developed for use as an exogenous chemical exchange dependent saturation transfer (CEST) contrast agent under physiological conditions. These agents would function by reducing the water proton signal through a chemical exchange site on the agent via saturation transfer. The ideal chemical exchange site would have a large chemical shift from water. This permits a high exchange rate without approaching the fast exchange limit at physiological pH (6.5-7.6) and temperature (37 degrees C), as well as minimizing problems associated with magnetic field susceptibility. Numerous candidate chemicals (amino acids, sugars, nucleotides, heterocyclic ring chemicals) were evaluated in this preliminary study. Of these, barbituric acid and 5, 6-dihydrouracil were more fully characterized with regard to pH, temperature, and concentration CEST effects. The best chemical exchange site found was the 5.33-ppm indole ring -NH site of 5-hydroxytryptophan. These data demonstrate that a CEST-based exogenous contrast agent for MRI is feasible.
Review of a new molecular imaging method——deuterium metabolic spectroscopy and imaging
[J].
分子影像新技术——氘代谢波谱及成像的综述与展望
[J].
DOI:10.11938/cjmr20222999
[本文引用: 1]

目前常用的分子影像技术主要有正电子发射型断层显像(PET)、质子磁共振波谱(<sup>1</sup>H MRS)及成像(<sup>1</sup>H MRSI)、化学交换饱和转移(CEST)、超极化<sup>13</sup>C MRSI等.近4年来,氘代谢波谱(DMS)及成像(DMI)作为一种新兴的分子影像技术获得了越来越多的关注,其通过采集注射或口服氘代葡萄糖后的目标组织与正常组织间氘代谢产物的磁共振信号进行组织区分.相比于其他分子影像方法,该影像技术具有无辐射、稳定性好、扫描操作相对简单等优点.本文综述了近年来DMS/DMI技术的研究进展及其意义,归纳总结了其临床应用价值,并对该技术未来的发展和改进方向,以及应用前景进行了展望.
Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST)
[J].Solution pH was measured using water proton NMR via chemical exchange dependent saturation transfer (CEST) with selected chemical exchange sites. Several useful pH-sensitive proton chemical exchange agents were found: 5,6-dihydrouracil, 5-hydroxytryptophan, and a combination of 5-hydroxytryptophan and 2-imidazolidinethione. A ratiometric approach was developed that permitted pH determinations that were independent of water T(1) or exchange site concentration.
Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI
[J].
DOI:10.1038/nm907
PMID:12872167
[本文引用: 3]

In the past decade, it has become possible to use the nuclear (proton, 1H) signal of the hydrogen atoms in water for noninvasive assessment of functional and physiological parameters with magnetic resonance imaging (MRI). Here we show that it is possible to produce pH-sensitive MRI contrast by exploiting the exchange between the hydrogen atoms of water and the amide hydrogen atoms of endogenous mobile cellular proteins and peptides. Although amide proton concentrations are in the millimolar range, we achieved a detection sensitivity of several percent on the water signal (molar concentration). The pH dependence of the signal was calibrated in situ, using phosphorus spectroscopy to determine pH, and proton exchange spectroscopy to measure the amide proton transfer rate. To show the potential of amide proton transfer (APT) contrast for detecting acute stroke, pH effects were noninvasively imaged in ischemic rat brain. This observation opens the possibility of using intrinsic pH contrast, as well as protein- and/or peptide-content contrast, as diagnostic tools in clinical imaging.
pH Imaging based on chemical exchange saturation transfer: Principles, methods, applications and recent progresses
[J].
基于CEST机制的pH成像方法、原理和应用
[J].
DOI:10.11938/cjmr20182664
[本文引用: 1]

化学交换饱和转移(Chemical Exchange Saturation Transfer,CEST)技术作为一种新型的磁共振成像(Magnetic Resonance Imaging,MRI)技术.它的原理为溶质池中被激发饱和的质子与游离水中未被饱和的质子间的化学交换,能够引起水质子磁共振信号的下降,从而获得组织内生物分子的相关信息.由于质子间的交换速率k<sub>ex</sub>与组织微环境的pH值之间存在直接联系,因而可以通过溶质质子的CEST信号对活体组织进行pH成像.目前用于pH成像的溶质分子既包括内源性游离的蛋白质、多肽分子,还包括一系列的外源性小分子和金属螯合物.通过不同类型的比率法、内源性胺和酰胺浓度-独立检测(Amine and Amide Concentration-independent Detection,AACID)等成像方法,能够获得肾脏、中风脑组织以及肿瘤组织的pH图谱.本文详细总结了2000年以来利用CEST技术进行pH成像方面的研究进展,包括对比剂、成像方法和相关应用,展望了活体组织pH成像的发展趋势和应用前景.
Ln(iii)-dotamgly complexes: A versatile series to assess the determinants of the efficacy of paramagnetic chemical exchange saturation transfer agents for magnetic resonance imaging applications
[J].Paramagnetic Ln-DOTAMGly complexes (Ln not equal La, Lu, and Gd) are the prototypes of a novel class of contrast agents for magnetic resonance imaging based on chemical exchange saturation transfer (CEST). Their ability to reduce the water signal intensity depends on the interplay of several physico-chemical properties of the agent and instrumental parameters. This study aims to identify possible routes for their optimizationSaturation transfer (ST) has been measured in vitro at 7.05 T as a function of pH, temperature, and concentration of the agent.Large saturation transfer effects have been observed upon irradiating the coordinated water protons (for Ln = Pr, Nd, Eu, and Tb). The comparison of the results obtained by irradiating water versus amide protons allows the set-up of ratiometric methods through which the ST response can be made independent on the concentration of the agent.The modulation of the magnetic properties along the lanthanide series allows an in-depth understanding of the determinants of ST effect and provides useful insights for the design of more efficient agents.
Saturation power dependence of amide proton transfer image contrasts in human brain tumors and strokes at 3 T
[J].
DOI:10.1002/mrm.22891
PMID:21394783
[本文引用: 2]

Amide proton transfer (APT) imaging is capable of detecting mobile cellular proteins and peptides in tumor and monitoring pH effects in stroke, through the saturation transfer between irradiated amide protons and water protons. In this work, four healthy subjects, eight brain tumor patients (four with high-grade glioma, one with lung cancer metastasis, and three with meningioma), and four stroke patients (average 4.3 ± 2.5 days after the onset of the stroke) were scanned at 3 T, using different radiofrequency saturation powers. The APT effect was quantified using the magnetization transfer ratio (MTR) asymmetry at 3.5 ppm with respect to the water resonance. At a saturation power of 2 μT, the measured APT-MRI signal of the normal brain tissue was almost zero, due to the contamination of the negative conventional magnetization transfer ratio asymmetry. This irradiation power caused an optimal hyperintense APT-MRI signal in the tumor and an optimal hypointense signal in the stroke, compared to the normal brain tissue. The results suggest that the saturation power of 2 μT is ideal for APT imaging of these two pathologies at 3 T with the existing clinical hardware.Copyright © 2011 Wiley-Liss, Inc.
Whole-brain amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging in glioma patients using low-power steady-state pulsed chemical exchange saturation transfer (CEST) imaging at 7T
[J].
Validation of the presence of fast exchanging amine CEST effect at low saturation powers and its influence on the quantification of APT
[J].
DOI:10.1002/mrm.29742
PMID:37317709
[本文引用: 1]

Accurately quantifying the amide proton transfer (APT) effect and the underlying exchange parameters is crucial for its applications, but previous studies have reported conflicting results. In these quantifications, the CEST effect from the fast exchange amine was always ignored because it was considered weak with low saturation powers. This paper aims to evaluate the influence of the fast exchange amine CEST on the quantification of APT at low saturation powers.A quantification method with low and high saturation powers was used to distinguish APT from the fast exchange amine CEST effect. Simulations were conducted to assess the method's capability to separate APT from the fast exchange amine CEST effect. Animal experiments were performed to assess the relative contributions from the fast exchange amine and amide to CEST signals at 3.5 ppm. Three APT quantification methods, each with varying degrees of contamination from the fast exchange amine, were employed to process the animal data to assess the influence of the amine on the quantification of APT effect and the exchange parameters.The relative size of the fast exchange amine CEST effect to APT effect gradually increases with increasing saturation power. At 9.4 T, it increases from approximately 20% to 40% of APT effect with a saturation power increase from 0.25 to 1 μT.The fast exchange amine CEST effect leads overestimation of APT effect, fitted amide concentration, and amide-water exchange rate, potentially contributing to the conflicting results reported in previous studies.© 2023 International Society for Magnetic Resonance in Medicine.
Clinical translation of amide proton transfer (APT) MRI for ischemic stroke: A systematic review (2003-2020)
[J].
Snapshot-CEST: Optimizing spiral-centric-reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T
[J].
Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging
[J].
DOI:10.1002/mrm.21712
PMID:18816868
[本文引用: 1]

Amide proton transfer (APT) imaging is a type of chemical exchange-dependent saturation transfer (CEST) magnetic resonance imaging (MRI) in which amide protons of endogenous mobile proteins and peptides in tissue are detected. Initial studies have shown promising results for distinguishing tumor from surrounding brain in patients, but these data were hampered by magnetic field inhomogeneity and a low signal-to-noise ratio (SNR). Here a practical six-offset APT data acquisition scheme is presented that, together with a separately acquired CEST spectrum, can provide B(0)-inhomogeneity corrected human brain APT images of sufficient SNR within a clinically relevant time frame. Data from nine brain tumor patients at 3T shows that APT intensities were significantly higher in the tumor core, as assigned by gadolinium-enhancement, than in contralateral normal-appearing white matter (CNAWM) in patients with high-grade tumors. Conversely, APT intensities in tumor were indistinguishable from CNAWM in patients with low-grade tumors. In high-grade tumors, regions of increased APT extended outside of the core into peripheral zones, indicating the potential of this technique for more accurate delineation of the heterogeneous areas of brain cancers.(c) 2008 Wiley-Liss, Inc.
3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T
[J].
DOI:10.1002/mrm.27569
PMID:30431179
[本文引用: 1]

For clinical implementation, a chemical exchange saturation transfer (CEST) imaging sequence must be fast, with high signal-to-noise ratio (SNR), 3D coverage, and produce robust contrast. However, spectrally selective CEST contrast requires dense sampling of the Z-spectrum, which increases scan duration. This article proposes a compromise: using a 3D snapshot gradient echo (GRE) readout with optimized CEST presaturation, sampling, and postprocessing, highly resolved Z-spectroscopy at 3T is made possible with 3D coverage at almost no extra time cost.A 3D snapshot CEST sequence was optimized for low-power CEST MRI at 3T. Pulsed saturation was optimized for saturation power and saturation duration. Spectral sampling and postprocessing (B correction, denoising) was optimized for spectrally selective Lorentzian CEST effect extraction. Reproducibility was demonstrated in 3 healthy volunteers and feasibility was shown in 1 tumor patient.Low-power saturation was achieved by a train of 80 pulses of duration t = 20 ms (total saturation time t = 3.2 seconds at 50% duty cycle) with B = 0.6 μT at 54 irradiation frequency offsets. With the 3D snapshot CEST sequence, a 180 × 220 × 54 mm field of view was acquired in 7 seconds per offset. Spectrally selective CEST effects at +3.5 and -3.5 ppm were quantified using multi-Lorentzian fitting. Reproducibility was high with an intersubject coefficient of variation below 10% in CEST contrasts. Amide and nuclear overhauser effect CEST effects showed similar correlations in tumor and necrosis as show in previous ultra-high field work.A sophisticated CEST tool ready for clinical application was developed and tested for feasibility.© 2018 International Society for Magnetic Resonance in Medicine.
High quality three-dimensional gagCEST imaging of in vivo human knee cartilage at 7 Tesla
[J].
DOI:10.1002/mrm.26265
PMID:27174078
[本文引用: 2]

To develop a new faster and higher quality three-dimensional (3D) gagCEST MRI technique for reliable quantification of glycosaminoglycan (GAG) present in the human knee cartilages.A new magnetization-prepared 3D gradient echo-based MRI pulse sequence has been designed to obtain the B inhomogeneity, B inhomogeneity, and CEST Z-spectra images.The gagCEST values of different compartments of knee cartilage are calculated using a newly developed technique for healthy subjects and a symptomatic knee cartilage degenerated subject. The effect of the acquired CEST saturation frequency offset step-size was investigated to establish the optimal step-size to obtain reproducible gagCEST maps. Our novel 3D gagCEST technique demonstrates markedly higher gagCEST contrast value than the previously reported 3D gagCEST studies. This study demonstrates the need for separate B and B inhomogeneity estimation and correction.The new technique provided high quality gagCEST maps with clearer visualization of different layers of knee cartilage with reproducible results. Magn Reson Med 77:1866-1873, 2017. © 2016 International Society for Magnetic Resonance in Medicine.© 2016 International Society for Magnetic Resonance in Medicine.
Whole-brain steady-state CEST at 3 T using MR multitasking
[J].
In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T
[J].
DOI:10.1002/mrm.23141
PMID:22083645
[本文引用: 1]

Chemical exchange saturation transfer (CEST) is a technique to indirectly detect pools of exchangeable protons through the water signal. To increase its applicability to human studies, it is needed to develop sensitive pulse sequences for rapidly acquiring whole-organ images while adhering to stringent amplifier duty cycle limitations and specific absorption rate restrictions. In addition, the interfering effects of direct water saturation and conventional magnetization transfer contrast complicate CEST quantification and need to be reduced as much as possible. It is shown that for protons exchanging with rates of less than 50-100 Hz, such as imaged in amide proton transfer experiments, these problems can be addressed by using a three-dimensional steady state pulsed acquisition of limited B(1) strength (≈ 1 μT). Such an approach exploits the fact that the direct water saturation width, magnetization transfer contrast magnitude, and specific absorption rate increase strongly with B(1), while the size of the CEST effect for such protons depends minimally on B(1). A short repetition time (65 ms) steady-state sequence consisting of a brief saturation pulse (25 ms) and a segmented echo-planar imaging train allowed acquisition of a three-dimensional whole-brain volume in approximately 11 s per saturation frequency, while remaining well within specific absorption rate and duty cycle limits. Magnetization transfer contrast was strongly reduced, but substantial saturation effects were found at frequencies upfield from water, which still confound the use of magnetization transfer asymmetry analysis. Fortunately, the limited width of the direct water saturation signal could be exploited to fit it with a Lorentzian function allowing CEST quantification. Amide proton transfer effects ranged between 1.5% and 2.5% in selected white and grey matter regions. This power and time-efficient 3D pulsed CEST acquisition scheme should aid endogenous CEST quantification at both high and low fields.Copyright © 2011 Wiley Periodicals, Inc.
Optimization of pulse train presaturation for CEST imaging in clinical scanners
[J].
DOI:10.1002/mrm.22750
PMID:21337418
[本文引用: 1]

Chemical exchange saturation transfer (CEST) imaging depends on the performance of radiofrequency saturation during the experiment. Scanner specifications, in particular limited pulse width and duty-cycle, and specific absorption rate guidelines restrict the full exploitation of CEST effects in clinical MR systems. The purpose of this study was to optimize techniques for effective pulse train presaturation for CEST imaging in a whole-body MR scanner. Theoretical analysis and simulations of the spectral properties of radiofrequency pulse trains demonstrated the significance of pulse width τ(P) and interpulse delay τ(D) for effective and selective labeling of a chemically exchanging proton pool. CEST experiments with model solutions, e.g., creatine dissolved in water, showed best performance of pulse trains with τ(P) = τ(D) = 100 msec, regarding minimum direct water saturation in z-spectra and distinct magnetization transfer ratio asymmetry that can be determined quantitatively. Saturation efficiency of trains of Gaussian-shaped radiofrequency pulses using this timing was evaluated in MR imagers with field strengths of 1.5, 3, and 7 T. The proposed saturation pulse train does not require hardware modifications, offers low specific absorption rate, and can be used in a standard clinical setup.Copyright © 2011 Wiley-Liss, Inc.
Fd-Net: An unsupervised deep forward-distortion model for susceptibility artifact correction in EPI
[J].
DOI:10.1002/mrm.29851
PMID:37811681
[本文引用: 1]

To introduce an unsupervised deep-learning method for fast and effective correction of susceptibility artifacts in reversed phase-encode (PE) image pairs acquired with echo planar imaging (EPI).Recent learning-based correction approaches in EPI estimate a displacement field, unwarp the reversed-PE image pair with the estimated field, and average the unwarped pair to yield a corrected image. Unsupervised learning in these unwarping-based methods is commonly attained via a similarity constraint between the unwarped images in reversed-PE directions, neglecting consistency to the acquired EPI images. This work introduces a novel unsupervised deep Forward-Distortion Network (FD-Net) that predicts both the susceptibility-induced displacement field and the underlying anatomically correct image. Unlike previous methods, FD-Net enforces the forward-distortions of the correct image in both PE directions to be consistent with the acquired reversed-PE image pair. FD-Net further leverages a multiresolution architecture to maintain high local and global performance.FD-Net performs competitively with a gold-standard reference method (TOPUP) in image quality, while enabling a leap in computational efficiency. Furthermore, FD-Net outperforms recent unwarping-based methods for unsupervised correction in terms of both image and field quality.The unsupervised FD-Net method introduces a deep forward-distortion approach to enable fast, high-fidelity correction of susceptibility artifacts in EPI by maintaining consistency to measured data. Therefore, it holds great promise for improving the anatomical accuracy of EPI imaging.© 2023 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Parallel EPI artifact correction (PEAC) for N/2 ghost suppression in neuroimaging applications
[J].
DOI:10.1016/j.mri.2013.03.021
PMID:23601363
[本文引用: 1]

Echo Planar Imaging (EPI) is a neuroimaging tool for clinical practice and research investigation. Due to odd-even echo phase inconsistencies, however, EPI suffers from Nyquist N/2 ghost artifacts. In standard neuroimaging protocols, EPI artifacts are suppressed using phase correction techniques that require reference data collected from a reference scan. Because reference-scan based techniques are sensitive to subject motion, EPI performance is sub-optimal in neuroimaging applications. In this technical note, we present a novel EPI data processing technique which we call Parallel EPI Artifact Correction (PEAC). By introducing an implicit data constraint associated with multi-coil sensitivity in parallel imaging, PEAC converts phase correction into a constrained problem that can be resolved using an iterative algorithm. This enables "reference-less" EPI that can improve neuroimaging performance. In the presented work, PEAC is investigated using a standard functional magnetic resonance imaging (fMRI) protocol with multi-slice 2D EPI. It is demonstrated that PEAC can suppress ghost artifacts as effectively as the standard reference-scan based phase correction technique used on a clinical MRI system. We also found that PEAC can achieve dynamic phase correction when motion occurs.Copyright © 2013 Elsevier Inc. All rights reserved.
FLASH imaging: Rapid NMR imaging using low flip-angle pulses. 1986
[J].DOI:10.1016/j.jmr.2011.09.021 PMID:22152368 [本文引用: 1]
Fast multislice pH-weighted chemical exchange saturation transfer (CEST) MRI with unevenly segmented RF irradiation
[J].
DOI:10.1002/mrm.22628
PMID:20872859
[本文引用: 1]

Chemical exchange saturation transfer (CEST) MRI is a versatile imaging technique for measuring microenvironment properties via dilute CEST labile groups. Conventionally, CEST MRI is implemented with a long radiofrequency irradiation module, followed by fast image acquisition to obtain the steady state CEST contrast. Nevertheless, the sensitivity, scan time, and spatial coverage of the conventional CEST MRI method may not be optimal. Our study proposed a segmented radiofrequency labeling scheme that includes a long primary radiofrequency irradiation module to generate the steady state CEST contrast and repetitive short secondary radiofrequency irradiation module immediately after the image acquisition so as to maintain the steady state CEST contrast for multislice acquisition and signal averaging. The proposed CEST MRI method was validated experimentally with a tissue-like pH phantom and optimized for the maximal contrast-to-noise ratio. In addition, the proposed sequence was evaluated for imaging ischemic acidosis via pH-weighted endogenous amide proton transfer MRI, which showed similar contrast as conventional amide proton transfer MRI. In sum, a fast multislice relaxation self-compensated CEST MRI sequence was developed, with significantly improved sensitivity and suitable for in vivo applications.Copyright © 2010 Wiley-Liss, Inc.
3D CEST MRI with an unevenly segmented RF irradiation scheme: A feasibility study in brain tumor imaging
[J].
DOI:10.1002/mrm.29810
PMID:37526017
[本文引用: 1]

To integrate 3D CEST EPI with an unevenly segmented RF irradiation module and preliminarily demonstrate it in the clinical setting.A CEST MRI with unevenly segmented RF saturation was implemented, including a long primary RF saturation to induce the steady-state CEST effect, maintained with repetitive short secondary RF irradiation between readouts. This configuration reduces relaxation-induced blur artifacts during acquisition, allowing fast 3D spatial coverage. Numerical simulations were performed to select parameters such as flip angle (FA), short RF saturation duration (Ts2), and the number of readout segments. The sequence was validated experimentally with data from a phantom, healthy volunteers, and a brain tumor patient.Based on the numerical simulation and l-carnosine gel phantom experiment, FA, Ts2, and the number of segments were set to 20°, 0.3 s, and the range from 4 to 8, respectively. The proposed method minimized signal modulation in the human brain images in the k direction during the acquisition and provided the blur artifacts-free CEST contrast over the whole volume. Additionally, the CEST contrast in the tumor tissue region is higher than in the contralateral normal tissue region.It is feasible to implement a highly accelerated 3D EPI CEST imaging with unevenly segmented RF irradiation.© 2023 International Society for Magnetic Resonance in Medicine.
Intraindividual difference between supraclavicular and subcutaneous proton density fat fraction is associated with cold-induced thermogenesis
[J].
Self-adapting multi-peak water-fat reconstruction for the removal of lipid artifacts in chemical exchange saturation transfer (CEST) imaging
[J].
DOI:10.1002/mrm.27859
PMID:31241219
[本文引用: 1]

Artifacts caused by strong lipid signals pose challenges in body chemical exchange saturation transfer (CEST) imaging. This study aimed to develop an accurate water-fat reconstruction method based on the multi-echo Dixon technique to remove lipid artifacts in CEST imaging.It is well known that fat has multiple spectral peaks. Furthermore, RF pulses in CEST preparation saturate each fat peak at different levels, complicating fat modeling. Therefore, a self-adapting multi-peak model (SMPM) is proposed to update relative amplitudes of fat peaks using numerical calculation. With the SMPM-based updating, nonlinear least-squares fitting combined with IDEAL (Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation) algorithms was used for water-fat reconstruction and B mapping. The proposed method was compared with the reported 3-point Dixon method and the fixed multi-peak model in a phantom study using a fat-free Z-spectrum obtained from MR spectroscopy acquisition as the ground truth. This method was also validated by in vivo experiments on human breast.In the phantom experiments, the Z-spectrum from the SMPM-based method agreed well with the fat-free Z-spectrum from CEST-PRESS (point-resolved spectroscopy), validating the effective removal of lipid artifacts, while a decrease or a rise that appeared at -3.5 ppm was observed in the Z-spectrum from the 3-point method and the FMPM-based method, respectively. In the in vivo experiments, no lipid artifacts were observed in the Z-spectrum or the amide CEST map from the SMPM-based method in the fibro-glandular region of the breast with high fat fractions.The SMPM-based method successfully removes lipid artifacts and significantly improves the accuracy of CEST contrast.© 2019 International Society for Magnetic Resonance in Medicine.
Deep neural network based CEST and AREX processing: Application in imaging a model of alzheimer's disease at 3 T
[J].
User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability
[J].
DOI:10.1016/j.neuroimage.2006.01.015
PMID:16545965
[本文引用: 1]

Active contour segmentation and its robust implementation using level set methods are well-established theoretical approaches that have been studied thoroughly in the image analysis literature. Despite the existence of these powerful segmentation methods, the needs of clinical research continue to be fulfilled, to a large extent, using slice-by-slice manual tracing. To bridge the gap between methodological advances and clinical routine, we developed an open source application called ITK-SNAP, which is intended to make level set segmentation easily accessible to a wide range of users, including those with little or no mathematical expertise. This paper describes the methods and software engineering philosophy behind this new tool and provides the results of validation experiments performed in the context of an ongoing child autism neuroimaging study. The validation establishes SNAP intrarater and interrater reliability and overlap error statistics for the caudate nucleus and finds that SNAP is a highly reliable and efficient alternative to manual tracing. Analogous results for lateral ventricle segmentation are provided.
Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments
[J].
DOI:10.1002/mrm.21873
PMID:19358232
[本文引用: 1]

Chemical exchange saturation transfer (CEST) is a contrast mechanism that exploits exchange-based magnetization transfer (MT) between solute and water protons. CEST effects compete with direct water saturation and conventional MT processes, and generally can only be quantified through an asymmetry analysis of the water saturation spectrum (Z-spectrum) with respect to the water frequency, a process that is exquisitely sensitive to magnetic field inhomogeneities. Here it is shown that direct water saturation imaging allows measurement of the absolute water frequency in each voxel, allowing proper centering of Z-spectra on a voxel-by-voxel basis independently of spatial B(0) field variations. Optimal acquisition parameters for this "water saturation shift referencing" (WASSR) approach were estimated using Monte Carlo simulations and later confirmed experimentally. The optimal ratio of the WASSR sweep width to the linewidth of the direct saturation curve was found to be 3.3-4.0, requiring a sampling of 16-32 points. The frequency error was smaller than 1 Hz at signal-to-noise ratios of 40 or higher. The WASSR method was applied to study glycogen, where the chemical shift difference between the hydroxyl (OH) protons and bulk water protons at 3T is so small (0.75-1.25 ppm) that the CEST spectrum is inconclusive without proper referencing.
Improved differentiation of low-grade and high-grade gliomas and detection of tumor proliferation using APT contrast fitted from Z-Spectrum
[J].
DOI:10.1007/s11307-017-1154-y
PMID:29313159
[本文引用: 1]

The purpose of the study is to demonstrate the value of quantitative amide proton transfer (APT) imaging for differentiating glioma grades and detecting tumor proliferation.This study included 32 subjects with 16 low-grade gliomas (LGG) and 16 high-grade gliomas (HGG) confirmed by histopathology. Chemical exchange saturation transfer (CEST) magnetic resonance imaging with APT weighting was performed on a 3 T scanner. After B correction, Z-spectra were fitted with Lorentzian functions corresponding to the upfield semi-solid magnetization transfer and nuclear overhauser enhancement (MT&NOE) effect, the direct saturation (DS) effect, and the downfield APT effect centered at around - 1.5, 0, and + 3.5 ppm, respectively. To compute the Z-spectral fitted APT (fitted_APT) in solid tumor tissue, double-peak histogram fitting of pixel MT&NOE effect from the whole tumor was used to remove necrosis regions. The fitted APT was then compared with the conventional APT based on magnetization transfer ratio asymmetry. Receiver operating characteristic (ROC) analysis was used to compare the performance between Z-spectral fitted contrasts and the con_APT for LGG versus HGG differentiation. Additionally, the correlations between the imaging contrasts (fitted_APT, con_APT, and fitted_MT&NOE) and Ki-67 labeling index for tumor proliferation were also evaluated.Z-spectral fitted_APT shows improved statistical power for differentiating HGG and LGG (7.58 ± 0.99 vs. 6.79 ± 1.05 %, p < 0.05) than con_APT (4.34 ± 0.95 vs. 4.05 ± 2.02 %, p > 0.05) in solid tumor tissues. Analyses of whole tumor, on the other hand, have less differentiating power for both fitted_APT (p from 0.032 to 0.08) and con_APT (p from 0.696 to 0.809). Similarly, based on ROC analyses, fitted_APT shows larger area under the curve (AUC = 0.723) than con_APT (AUC = 0.543). The combination of fitted APT, DS, and MT&NOE further improved the specificity (75 %), diagnostic accuracy (78.2 %), and area under the curve (0.758) in differentiating LGG and HGG. Consistently, fitted_APT (r = 0.451, p = 0.018) is better correlated with Ki-67 than con_APT (r = 0.331, p = 0.092).Fitted APT from Z-spectrum improves differentiation of low- and high-grade gliomas and better correlated with tumor proliferation than conventional APT.
Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI
[J].
DOI:10.1002/nbm.3048
PMID:24288260
[本文引用: 1]

Chemical exchange saturation transfer (CEST)-derived, pH-weighted, amide proton transfer (APT) MRI has shown promise in animal studies for the prediction of infarction risk in ischemic tissue. Here, APT MRI was translated to patients with acute stroke (1-24 h post-symptom onset), and assessments of APT contrast, perfusion, diffusion, disability and final infarct volume (23-92 days post-stroke) are reported. Healthy volunteers (n = 5) and patients (n = 10) with acute onset of symptoms (0-4 h, n = 7; uncertain onset <24 h, n = 3) were scanned with diffusion- and perfusion-weighted MRI, fluid-attenuated inversion recovery (FLAIR) and CEST. Traditional asymmetry and a Lorentzian-based APT index were calculated in the infarct core, at-risk tissue (time-to-peak, TTP; lengthening) and final infarct volume. On average (mean ± standard deviation), control white matter APT values (asymmetry, 0.019 ± 0.005; Lorentzian, 0.045 ± 0.006) were not significantly different (p > 0.05) from APT values in normal-appearing white matter (NAWM) of patients (asymmetry, 0.022 ± 0.003; Lorentzian, 0.048 ± 0.003); however, ischemic regions in patients showed reduced (p = 0.03) APT effects compared with NAWM. Representative cases are presented, whereby the APT contrast is compared quantitatively with contrast from other imaging modalities. The findings vary between patients; in some patients, a trend for a reduction in the APT signal in the final infarct region compared with at-risk tissue was observed, consistent with tissue acidosis. However, in other patients, no relationship was observed in the infarct core and final infarct volume. Larger clinical studies, in combination with focused efforts on sequence development at clinically available field strengths (e.g. 3.0 T), are necessary to fully understand the potential of APT imaging for guiding the hyperacute management of patients.Copyright © 2013 John Wiley & Sons, Ltd.
Fast 3D chemical exchange saturation transfer (CEST) imaging of the human brain
[J].
DOI:10.1002/mrm.22546
PMID:20632402
[本文引用: 1]

Chemical exchange saturation transfer magnetic resonance imaging can detect low-concentration compounds with exchangeable protons through saturation transfer to water. This technique is generally slow, as it requires acquisition of saturation images at multiple frequencies. In addition, multislice imaging is complicated by saturation effects differing from slice to slice because of relaxation losses. In this study, a fast three-dimensional chemical exchange saturation transfer imaging sequence is presented that allows whole-brain coverage for a frequency-dependent saturation spectrum (z-spectrum, 26 frequencies) in less than 10 min. The approach employs a three-dimensional gradient- and spin-echo readout using a prototype 32-channel phased-array coil, combined with two-dimensional sensitivity encoding accelerations. Results from a homogenous protein-containing phantom at 3T show that the sequence produced a uniform contrast across all slices. To show translational feasibility, scans were also performed on five healthy human subjects. Results for chemical exchange saturation transfer images at 3.5 ppm downfield of the water resonance, so-called amide proton transfer images, show that lipid signals are sufficiently suppressed and artifacts caused by B(0) inhomogeneity can be removed in postprocessing. The scan time and image quality of these in vivo results show that three-dimensional chemical exchange saturation transfer MRI using gradient- and spin-echo acquisition is feasible for whole-brain chemical exchange saturation transfer studies at 3T in a clinical time frame.2010 Wiley-Liss, Inc.
Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly-L-lysine and a starburst dendrimer
[J].The ability to measure proton exchange rates in tissue using MRI would be very useful for quantitative assessment of magnetization transfer properties, both in conventional MT imaging and in the more recent chemical exchange saturation transfer (CEST) approach. CEST is a new MR contrast mechanism that depends on several factors, including the exchange rate of labile protons in the agent in a pH-dependent manner. Two new methods to monitor local exchange rate based on CEST are introduced. The two MRI-compatible approaches to measure exchange are quantifying exchange using saturation time (QUEST) dependence and quantifying exchange using saturation power (QUESP) dependence. These techniques were applied to poly-L-lysine (PLL) and a generation-5 polyamidoamine dendrimer (SPD-5) to measure the pH dependence of amide proton exchange rates in the physiologic range. Data were fit both to an analytical expression and to numerical solutions to the Bloch equations. Results were validated by comparison with exchange rates determined by two established spectroscopic methods. The exchange rates determined using the four methods were pooled for the pH-calibration curve of the agents consisting of contributions from spontaneous (k0) acid catalyzed (ka), and base catalyzed (kb) exchange rate constants. These constants were k0 = 68.9 Hz, ka = 1.21 Hz, kb = 1.92 x 10(9) Hz, and k0 = 106.4 Hz, ka = 25.8 Hz, kb = 5.45 x 10(8) Hz for PLL and SPD-5, respectively, showing the expected predominance of base-catalyzed exchange for these amide protons.(c) 2006 Wiley-Liss, Inc.
QUESP and QUEST revisited-fast and accurate quantitative CEST experiments
[J].
Understanding quantitative pulsed CEST in the presence of MT
[J].
DOI:10.1002/mrm.23074
PMID:21858864
[本文引用: 2]

Phantom experiments in agar and ammonium chloride were performed to evaluate a three-pool model of magnetization transfer and chemical exchange saturation transfer (CEST) in a pulsed saturation transfer experiment. The utility of the pulsed CEST method was demonstrated by varying the pH of the phantoms and observing the effect upon the CEST spectra both with and without the solid agar (the magnetization transfer pool), while fitting the spectra to the Bloch equation model with exchange. Pulsed CEST could be used to robustly quantify parameters related to CEST, including the exchange rate constant describing proton exchange with free water and the concentration of exchanging protons. Furthermore, the exchange rate constant and the CEST pool offset frequency of the ammonium chloride remained unchanged in the presence of a magnetization transfer pool. The logarithm of the fitted exchange rate constant was linearly related to pH: this relationship was maintained in the presence of magnetization transfer.Copyright © 2011 Wiley-Liss, Inc.
Quantitative chemical exchange saturation transfer (qCEST) MRI-RF spillover effect-corrected omega plot for simultaneous determination of labile proton fraction ratio and exchange rate
[J].
Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI
[J].
Nuclear overhauser enhancement (NOE) imaging in the human brain at 7 T
[J].
Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 Tesla
[J].
Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors
[J].
DOI:10.1002/mrm.29241
PMID:35452155
[本文引用: 1]

Amide proton transfer-weighted (APTw) MR imaging shows promise as a biomarker of brain tumor status. Currently used APTw MRI pulse sequences and protocols vary substantially among different institutes, and there are no agreed-on standards in the imaging community. Therefore, the results acquired from different research centers are difficult to compare, which hampers uniform clinical application and interpretation. This paper reviews current clinical APTw imaging approaches and provides a rationale for optimized APTw brain tumor imaging at 3 T, including specific recommendations for pulse sequences, acquisition protocols, and data processing methods. We expect that these consensus recommendations will become the first broadly accepted guidelines for APTw imaging of brain tumors on 3 T MRI systems from different vendors. This will allow more medical centers to use the same or comparable APTw MRI techniques for the detection, characterization, and monitoring of brain tumors, enabling multi-center trials in larger patient cohorts and, ultimately, routine clinical use.© 2022 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
DeepCEST 3T: Robust MRI parameter determination and uncertainty quantification with neural networks-application to CEST imaging of the human brain at 3T
[J].
DOI:10.1002/mrm.28117
PMID:31821616
[本文引用: 1]

Calculation of sophisticated MR contrasts often requires complex mathematical modeling. Data evaluation is computationally expensive, vulnerable to artifacts, and often sensitive to fit algorithm parameters. In this work, we investigate whether neural networks can provide not only fast model fitting results, but also a quality metric for the predicted values, so called uncertainty quantification, investigated here in the context of multi-pool Lorentzian fitting of CEST MRI spectra at 3T.A deep feed-forward neural network including a probabilistic output layer allowing for uncertainty quantification was set up to take uncorrected CEST-spectra as input and predict 3T Lorentzian parameters of a 4-pool model (water, semisolid MT, amide CEST, NOE CEST), including the B inhomogeneity. Networks were trained on data from 3 subjects with and without data augmentation, and applied to untrained data from 1 additional subject and 1 brain tumor patient. Comparison to conventional Lorentzian fitting was performed on different perturbations of input data.The deepCEST 3T networks provided fast and accurate predictions of all Lorentzian parameters and were robust to input perturbations because of noise or B artifacts. The uncertainty quantification detected fluctuations in input data by increase of the uncertainty intervals. The method generalized to unseen brain tumor patient CEST data.The deepCEST 3T neural network provides fast and robust estimation of CEST parameters, enabling online reconstruction of sophisticated CEST contrast images without the typical computational cost. Moreover, the uncertainty quantification indicates if the predictions are trustworthy, enabling confident interpretation of contrast changes.© 2019 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
CEST imaging of the abdomen with neural network fitting
[J].
基于神经网络拟合的腹部化学交换饱和转移成像
[J].
DOI:10.11938/cjmr20212903
[本文引用: 1]

磁共振成像(Magnetic Resonance Imaging,MRI)化学交换饱和转移(Chemical Exchange Saturation Transfer,CEST)技术在临床诊断中展现了巨大的潜力,但在腹部成像中受到主磁场偏移量大的挑战,而且利用传统的非对称性分析法得到的酰胺质子转移(Amide Proton Transfer,APT)成像对比度受到核奥氏增强(Nuclear Overhauser Enhancement,NOE)效应的干扰.本文提出了一种基于神经网络拟合的CEST后处理方法,对每个像素采集得到的Z谱特征进行识别,不需要额外序列扫描即可得到背景参考Z谱与主磁场偏移量,用以校正和获得理想的Z谱,并进一步分离得到源自APT效应与NOE效应的信号.鸡蛋清和健康志愿者腹部成像结果显示,本文提出的基于神经网络的CEST后处理方法效果较好.
Fast multi-channel magnetic resonance imaging based on PCAU-Net
[J].
基于PCAU-Net的快速多通道磁共振成像方法
[J].
DOI:10.11938/cjmr20222992
[本文引用: 1]

多通道磁共振成像方法采用多个接收线圈同时欠采样k空间以加快成像速度,并基于后处理算法重建图像,但在较高加速因子时,其图像重建质量仍然较差.本文提出了一种基于PCAU-Net的快速多通道磁共振成像方法,将单通道实数U型卷积神经网络拓展到多通道复数卷积神经网络,设计了一种结构不对称的U型网络结构,通过在解码部分减小网络规模以降低模型的复杂度.PCAU-Net网络在跳跃连接前增加了1×1卷积,以实现跨通道信息交互.输入和输出之间利用残差连接为误差的反向传播提供捷径.实验结果表明,使用规则和随机采样模板,在不同加速因子时,相比常规的GRAPPA重建算法和SPIRiT重建方法,本文提出的PCAU-Net方法可高质量重建出磁共振复数图像,并且相比于PCU-Net方法,PCAU-Net减少了模型参数、缩短了训练时间.
Magnetic resonance image reconstruction of multi-scale residual Unet fused with attention mechanism
[J].
融合注意力机制的多尺度残差Unet的磁共振图像重建
[J].
DOI:10.11938/cjmr20223040
[本文引用: 1]

为了提高磁共振图像在欠采样下重建的质量,本文融合注意力机制和多尺度残差卷积构建Unet网络,实现磁共振图像在欠采样下的重建算法.为增强网络特征的表现能力,以及防止网络训练中梯度消失与退化的问题,在Unet网络的编码路径中引入多尺度残差卷积,提取不同尺度的特征信息;为能准确地恢复图像的细节纹理特征,在Unet网络编码和解码路径的跳层拼接部分引入卷积注意力块,对细节纹理等关键信息进行不同程度的响应.实验表明,本文方法可通过欠采样k-空间数据快速重建出细节纹理清晰且无重叠伪影的高质量磁共振图像.
Assessment of frequency drift on CEST MRI and dynamic correction: Application to gagCEST at 7 T
[J].
DOI:10.1002/mrm.27367
PMID:29851141
[本文引用: 1]

To investigate the effect of a frequency drift of the static magnetic field on 3D CEST MRI based on glycosaminoglycans (GAGs) of articular cartilage at 7 T and to introduce a retrospective correction method that uses the phase images of the gradient-echo readout.Repeated gagCEST and B measurements were performed in a glucose model solution and in vivo in the knee joint of 3 healthy volunteers at 7 T. Phase images of the modified 3D rectangular spiral centric-reordered gradient-echo CEST sequence were used to quantify and compensate the apparent frequency drift in repeated gagCEST measurements.The frequency drift of the MRI scanner strongly influences the gagCEST signal in the articular cartilage of the human knee joint. The gagCEST signal in the articular cartilage is changed by 0.18%/Hz while an average drift of 0.7 ± 0.2 Hz/minute was observed. The proposed correction method can be applied retrospectively without the need of additional measurements and provides improved comparability and reproducibility for gagCEST studies. This correction method may also be of interest for other applications of CEST MRI.Prospective or retrospective correction of the frequency drift of the MRI scanner is essential for reproducible gagCEST measurements. The proposed retrospective correction method fulfills this requirement without the need of additional measurements.© 2018 International Society for Magnetic Resonance in Medicine.
Spin-echo T1-weighted imaging of the brain with interleaved acquisition and presaturation pulse at 3 T: A feasibility study before clinical use
[J].
DOI:10.1016/j.acra.2008.12.026
PMID:19375955
[本文引用: 1]

Although spin-echo (SE) sequence has some advantages over gradient-echo sequence in brain imaging, gradient-echo sequence is commonly used for T1-weighted imaging (T1WI) at 3 T because contrast on SE T1WI is widely believed to be poor at 3 T. Recently, gray-white matter contrast on single-slice and multi-slice SE imaging with interslice gap was reported as better at 3 T than at 1.5 T. This study examined the feasibility of interleaved SE T1WI of the brain at 3 T. This study also examined whether presaturation pulse (PP) sufficiently suppresses intra-arterial signals because these signals tend to be hyperintense due to longer T1 at 3 T.Subjects consisted of 18 healthy volunteers. Two sets of T1WI were performed using SE sequence. One set consisted of imaging without PP, and the other consisted of imaging with PP. Each set contained three types of gapless imaging as follows; sequential, 100% interleaved, and 200% interleaved imaging. In each subject, contrast-to-noise ratio between gray-matter and white-matter (CNR(GM-WM)) and intra-arterial signals were evaluated.CNR(GM-WM) was significantly higher on interleaved images than on sequential images, regardless of PP (P <.0001). PP sufficiently suppressed intra-arterial signals (P <.0001).CNR(GM-WM) on SE T1WI at 3 T can be improved by interleaved acquisition, and PP sufficiently suppressed intra-arterial signals. Interleaved SE T1WI with PP appears clinically feasible at 3 T.
Assignment of molecular origins of NOE signal at -3.5 ppm in the brain
[J].
Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain
[J].Magnetization transfer (MT) imaging provides a unique method of tissue characterization by capitalizing on the interaction between solid-like tissue components and bulk water. We used a continuous-wave (CW) MT pulse sequence with low irradiation power to study healthy human brains in vivo at 3 T and quantified the asymmetry of the MT effects with respect to the water proton frequency. This asymmetry was found to be a difference of approximately a few percent from the water signal intensity, depending on both the RF irradiation power and the frequency offset. The experimental results could be quantitatively described by a modified two-pool MT model extended with a shift contribution for the semisolid pool with respect to water. For white matter, this shift was fitted to be 2.34 +/- 0.17 ppm (N = 5) upfield from the water signal.