引言
磁共振弹性成像(magnetic resonance elastography,MRE),是一种通过记录剪切波动在软组织中的传递状态,基于波动位移推算软组织生物力学特性参数的成像方法[7⇓-9].在一定的振动频率下,通过对组织内氢原子自旋相位的记录,MRE可以实现对组织的波动位移开展成像.通过观察组织的生物力学特性,并基于本构方程假设,对剪切波在组织中传播的波动方程进行逆向求解,可以得到表征组织生物力学特性的本构参量[10,11].目前,MRE在肝硬化的临床诊断分级上应用较多[8],并在欧美国家开展了较为成熟的临床应用[12].由于常规成像方法难以直接对脑组织的生物力学参量开展测量,现有基于结构像,或扩散加权成像(diffusion weighted imaging,DWI)[13]等间接判断组织力学特性方法较不稳定,且单纯统计相关性并不能准确反映空间分布差异性较大的力学参量.因此,MRE为脑组织力学参量的直接准确测量提供了可靠且有效的手段.
1 MRE概述
1.1 成像原理
通过在指定空间方向和时间点加载位移编码梯度,相应的波动位移的分量随时间的变化可以通过相位积累的方式记录下来[16].位移编码梯度所记录的累计相位为:
其中γ是磁旋比,ω是振动角频率,N是编码梯度所记录的波动周期个数,
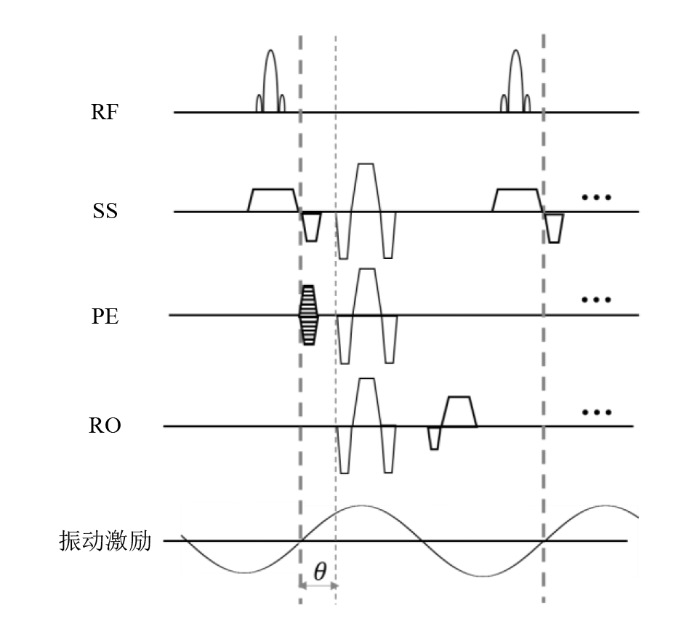
图1
图1
基于GRE的MRE序列示意. 在常规梯度回波的成像基础上,可以分别在SS、PE、RO方向加载位移编码梯度,从而对所加载方向的位移进行编码. SS:扫描层面选择;PE:相位编码;RO:读出梯度;θ:所施加的位移编码梯度与波动的相位差
Fig. 1
A typical MRE sequence based on gradient-echo imaging. Motion-encoding gradients can be applied to either SS, PE, or RO directions based on conventional gradient-echo sequence. SS: slice selection; PE: phase encoding; RO: readout; θ: phase offset between the motion encoding gradient and the harmonic motion
1.2 反演算法
剪切硬度μ可以基于组织密度ρ和剪切波动传播速度Cs进行估计:
对于
其中λ是拉梅系数,μ是剪切硬度,u是位移场,ρ是组织密度,是微分算子,t是时间变量.对于仅有剪切波动位移的情况,剪切位移的散度为0,上式简化为:
其通解为:
其中
使用相似定律将μ用
除了基于波动方程的直接反演计算(direct inversion,DI),常用的生物力学参量估计算法还包括本地频率估计(local frequency estimation,LFE)[25,26],多频率粘弹反算(k-multifrequency dual elasto-visco inversion,k-MDEV)[27],基于有限元模型的非线性反算(nonlinear inversion,NLI)[28],以及基于神经网络和深度学习(deep learning,DL)的算法[29,30].这些算法可以针对复数剪切模量
基于上述讨论,可以看到组织的粘弹性参量的表达方式多样,基于不同的模型与计算方法虽各有不同,但物理意义是相通的.其中,μ和
1.3 扫描流程
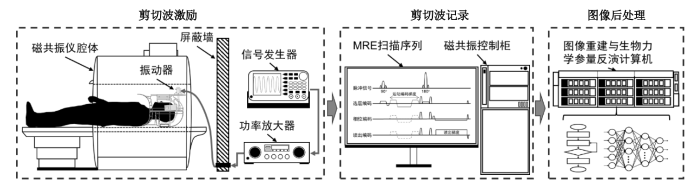
图2
图2
脑组织MRE成像的主要流程.剪切波激励包括波动信号的发生、放大与通过驱动器向脑组织中的传递;剪切波记录包括采用MRE扫描序列对磁共振成像系统操作记录波动位移;图像后处理部分包括图像的重建与基于波动影像的生物力学参量反演计算
Fig. 2
Procedures of brain MRE. The wave actuation includes generating, amplifying, and transmitting shear waves to brain. The recording of waves include using MRE sequence implemented in the MR scanner for displacement encoding. The image processing steps include image reconstruction and inversion of biomechanical properties
将剪切波动安全、有效并且舒适地传递到脑组织中,是MRE有效开展的首要步骤.当前,基于气动驱动的MRE成像驱动装置已在临床研究中广泛应用[39].气动驱动装置分为主动驱动和被动驱动两部分.其中主动驱动部包括产生气压的气泵和定频率控制系统,被动驱动部分由一个放置在头部下方的气枕组成,气枕和气泵由导气管连接,实现定频率变化的气压从主动驱动部分传递到被动驱动的气枕中.气动驱动器的磁兼容性能较好,气枕的布置和安放也比较灵活.另外一种传动方式采用刚性连接,将主动驱动与一个套在头上的摇盔固连,将产生的振动通过刚性的杆件和摇盔传递到脑中[40].类似的驱动方式还包括压电驱动,通过压电驱动器振动放置在胸前的硬质橡胶并通过人体传递到脑中[41].电磁驱动方法是利用磁共振成像的主磁场,通过在磁体中放置线圈并调节线圈中的电流,从而基于电磁感应产生振动[34].电磁驱动器可以产生较精准的频率驱动,较易实现振动的精准调控.
实际临床扫描过程中,被试者将驱动器穿戴好后,既可以开展常规扫描和MRE成像.目前基于EPI和GRE的MRE序列在临床较多使用.对于生物力学参量的反演计算,DI、k-MDEV、NLI和DL等算法均有应用.由于脑组织MRE尚集中在基础与临床研究中,其后处理算法依然在不断改进和更新.
2 MRE在脑疾病中的应用
由于测试频率和反演算法的差异,MRE测量的正常脑组织的剪切模量幅值在不同测试系统中不完全一致,但是对组织硬度变化的测量趋势是一致的.脑组织MRE常用测量频率为50~60 Hz,组织振动幅值在5~50 μm范围内[42,43].在此频率范围内,健康人正常全脑剪切模量幅值2.1~2.9 kPa [42],其中白质2.8~3.3 kPa,灰质2.0~2.4 kPa [44]. 脑组织的硬度随年龄增加而减小,大脑组织、白质和皮质灰组织的
2.1 脑肿瘤
表1 MRE在脑肿瘤中的研究小结.所有研究均在3 T磁共振系统上开展
Table 1
| 文献 | 肿瘤类型 | 患者总数(分布) | 驱动器/序列 | 分辨率/mm3 | 反演算法 | 测量结果/kPa* | |
|---|---|---|---|---|---|---|---|
| [46] | 脑膜瘤(4)、血管外皮细胞瘤(1)、神经鞘瘤(1) | 6(2男/4女, 16~63岁) | 电磁式驱动 咬棒/GRE | 1.875×1.875×5 | - | 肿瘤组织剪切模量幅值与手术直观判断相符 | |
| [47] | 脑膜瘤(13) | 13 | 气动枕/ SE-EPI | 4×4×4 | DI | 肿瘤组织剪切模量幅值与手术直观判断相符 | |
| [48] | 淋巴瘤(1)、胶质母细胞瘤(3)、间变性星形细 瘤(3)、神经胶质 瘤(4)、脑膜瘤(2)、脑转移瘤(3) | 16(5男/11女, 26~78岁) | 头部摇篮/ EPI | 3×3×3 | MDEV | |G*| = 0.893~2.131 | |
| [49] | 脑膜瘤(14) | 14(4男/10女, 28~76岁) | 气动枕/ SE-EPI | 3×3×3 | DI | 肿瘤组织剪切模量幅值与手术直观判断相符 | |
| [50] | 垂体大腺瘤(10) | 10(5男/5女, 22~78岁) | 气动枕/ SE-EPI | 3×3×3 | DI | 软肿瘤平均剪切模量幅值 1.38±0.36 (1.08~1.86) 硬肿瘤平均剪切模量幅值 1.94±0.26 (1.72~2.32) | |
| [51] | 神经胶质瘤(18) | 18(12男/6女, 男性25~68岁, 女性28~40) | 气动枕/ SE-EPI | 3×3×3 | DI | 平均剪切模量幅值 2.2±0.7 (1.1~3.8) | |
| [52] | 垂体腺瘤(38) | 38(22男/16女, 22~78岁) | 气动枕/ SE-EPI | 3×3×3 | DI | 平均剪切模量幅值 1.8 (1.1~3.7) | |
| [53] | 脑膜瘤(13)、垂体腺瘤(11)、前庭神经鞘瘤(6)、胶质瘤(4) | 34(11男/23女, 31~77岁) | 气动枕/ SE-EPI | 3.75 | DI | 平均剪切模量幅值 脑膜瘤1.9±0.8,垂体腺瘤1.2±0.3,前庭神经鞘瘤2.0±0.4,胶质瘤1.5±0.2 | |
| [54] | 脑膜瘤(18) | 18(4男/14女, 62.8±15.3岁) | 气动枕/ SE-EPI | 1.875×1.875×3 | DI | 平均剪切模量幅值 3.12±1.23 | |
| [55] | 脑膜瘤(88) | 88(35男/53女, 22~77岁) | 气动枕/ SE-EPI | 3×3×3 | DI | 平均剪切模量幅值 3.81±1.74 (1.57~12.6) | |
| [56] | 胶质母细胞瘤(22) | 22(12男/10女, 64.5±15.1岁) | 头部摇篮/ SE-EPI | 2×2×2 | MEDV | |G*| = 0.85~1.83 (1.32±0.26) | |
| [57] | 胶质母细胞瘤(11)、间变性星形细胞瘤(3)、脑膜瘤(7)、脑转移瘤(5)、脑内脓肿(1) | 27(12男/15女, 49~75岁) | 头部摇篮/ SE-EPI | 2×2×2 | MEDV | |G*| = 1.43±0.33 | |
| [58] | 转移瘤(1)、胶质母细胞瘤(3)、星形细胞瘤(1)、脑膜瘤(3) | 8(3男/5女, 28~76岁) | 头部摇篮/ SE-EPI | 2×2×2 | MEDV | 脑膜瘤和星形细胞瘤 |G*| = 1.52±0.20; 胶质瘤和转移瘤 |G*| = 1.28±0.14 | |
| [59] | 胶质母细胞瘤(10) | 10(5男/5女, 44~74岁) | 机械转子/ GRE | 3.1×3.1×3.1 | NLI | G° = 1.15~1.62; G = 0.55~0.80 | |
*测量的结果中,括号内表示最小值和最大值.
梅奥医学中心基于其开发的气动枕式驱动器,针对脑疾病开展了大量研究.其中,Hughes等人对脑膜瘤和垂体腺瘤进行了在体测量,验证了在体测量与开颅后术中判断的一致性[49],并发现软肿瘤和硬肿瘤的平均剪切模量幅值(最大~最小值)分别为1.38±0.36 (1.08~1.86) kPa和1.94±0.26 (1.72~2.32) kPa[50].Pepin等人对胶质瘤开展了测量,发现其平均剪切模量幅值为2.2±0.7 (1.1~3.8) kPa.对应II、III和IV级胶质瘤,其剪切模量幅值呈现下降趋势,平均值分别为2.7±0.7 (2.1~3.8) kPa、2.2±0.6 (1.7~3.4) kPa和1.7±0.5 (1.3~2.1) kPa[51]. Cohen-Cohen等人对垂体腺瘤测量发现其平均剪切模量幅值为1.8 kPa,比正常脑白质软[52].日本Sakai等人采用梅奥医学中心的系统,针对四种脑肿瘤开展测量得到其剪切模量幅值的平均值分别为:脑膜瘤1.9±0.8 kPa,垂体腺瘤1.2±0.3 kPa,前庭神经鞘瘤2.0±0.4 kPa,胶质瘤1.5±0.2 kPa[53].日本的Takamura等人与梅奥医学中心合作,采用平面分辨率为1.875 mm的MRE对18例脑膜瘤进行了测量并测得平均剪切模量幅值为3.12 ±1.23 kPa[54].中国医科大学附属盛京医院石喻等人与梅奥医学中心合作,采集了88例脑膜瘤并测得平均剪切模量幅值3.81±1.74 (1.57~12.6) kPa,比正常大脑和小脑组织都要硬[55].纵观如上梅奥医学中心的系列研究,针对脑膜瘤的测量最多,但所测量的剪切模量幅值并不完全一致.究其原因,相较于较早期开展的测量,后期测量所用的反演算法、扫描序列都有一定程度的改进,可能导致了早期和后期测量的绝对测量数值的变化.
德国Simon等人基于其开发的脑组织MRE成像系统,对淋巴瘤、胶质母细胞瘤、间变性星形细胞瘤、神经胶质瘤、脑膜瘤、脑转移瘤等一系列脑肿瘤开展了早期测量[48].其中,针对胶质瘤的测量发现其平均
2.2 神经退行性疾病
神经退行性疾病如阿尔茨海默症(Alzheimer’s disease,AD)和帕金森症(Parkinson’s disease,PD)是影响人类健康的主要脑疾病.表2总结了MRE在神经退行性疾病中的主要研究.MRE在这一领域的早期研究主要基于人脑的正常衰老开展测量,发现组织硬度随衰老下降的趋势.2009年德国Sack等人最早采用MRE方法开展对不同年龄人群的脑组织生物力学参数进行测量.他们基于对55个健康志愿者(18~88岁)的研究,发现脑组织的剪切模量幅值随着年龄增长下降的趋势[60].美国梅奥医学中心的Arani等人通过对来自不同年龄段的45名健康志愿者(56~89岁)开展全脑MRE测量,发现在除小脑之外的大脑(额叶、顶叶、颞叶和枕叶)区域,其剪切模量幅值均表现出随年龄增加而显著下降的现象[61].Hiscox等人对同等数量(12人)的青年组和老年组开展脑组织MRE测量,发现对比青年组,老年组的皮质下区域有显著的软化特性[31]. 美国斯坦福大学的Lv等人通过对46名健康志愿者(26~76岁)开展全脑MRE的测量,发现脑组织的粘弹参数,包括储能剪切模量,损耗剪切模量等均随着年龄的增加而下降,并发现尾状核、壳核和丘脑的剪切模量可以用于标志衰老,且年龄越大其组织软化的程度越大[45].美国特拉华大学的Delgorio等人通过对54个健康志愿者(21~81岁)的海马和海马亚区的组织硬度开展测量和分析,发现海马区及其亚区的硬度随着年龄显著下降[62].近期他们的一项针对遗忘型轻度认知障碍患者的研究也有类似发现[63].
表2 MRE在神经退行性疾病中的研究小结.所有研究均在3 T磁共振系统上开展
Table 2
| 文献 | 疾病类型 (患者数量) | 患者年龄 (岁) | 驱动器/ 序列 | 分辨率/ mm3 | 反演算法 | 测量结果/kPa* | 所测量参量显著变化 的区域 |
|---|---|---|---|---|---|---|---|
| [63] | 遗忘型轻度认知 障碍(20) | 23~81 | 气动枕/ 3D spiral | 1.25×1.25×1.25 | NLI | 对照组:2.84±0.28 遗忘型轻度认知障碍: 2.63±0.32 | 阿蒙尼角1-2,齿状回-阿蒙尼角3 |
| [64] | 阿尔茨海默症(7) | 76~94 | 气动枕/ SE-EPI | 4×4×2.5 | DI | CN-: 2.37 (2.17~2.62); CN+: 2.32 (2.18~2.67); AD: 2.20 (1.96~2.29) | 全脑 |
| [65] | 阿尔茨海默症(8) | - | 气动枕/ SE-EPI | 3×3×3 | DI | 对照组:2.51±0.09 疾病组:2.40±0.09 | 额叶,颞叶以及一个复合区域(额叶、顶叶和颞叶,不包括中央前回和中央后回) |
| [66] | 阿尔茨海默症(8) 路易体痴呆(13) 额颞叶痴呆(5) | 78~88 55~79 54~65 | 气动枕/ SE-EPI | 3×3×3 | NLI | 对照组:2.81±0.22 阿尔茨海默症:2.35±0.26 路易体痴呆:2.84±0.30 额颞叶痴呆:2.30±0.20 | 阿尔茨海默症:额叶、颞叶、扣带回、辅助运动区、楔前叶和眶前区 路易体痴呆:楔前叶 额颞叶痴呆:顶叶、额叶、颞叶、中央前叶、枕盖、岛叶、楔前叶、眶前叶、初级视觉区、扣带回和枕叶 |
| [67] | 阿尔茨海默症(21) | 67~80 | 头部摇篮/ SE-EPI | 1.9×1.9×1.9 | MDEV | 对照组:1.54±0.13 阿尔茨海默症:1.39±0.16 | 海马区 |
| [68] | 阿尔茨海默症(12) | 70~87 | 气动枕/ 3D spiral | 1.6×1.6×1.6 | NLI | 对照组:2.50±0.05 阿尔茨海默症:2.25±0.05 | 白质、皮质灰质(颞中上回和楔前叶) |
| [69] | 帕金森症(17) 进行性核上性麻痹 (20) | 49~78 62~82 | 头部摇篮/ SE-EPI | 2×2×2 | MDEV | 对照组:1.04±0.08 帕金森症:0.96±0.065 进行性核上性麻痹: 0.95±0.078 | 帕金森症:额叶和中脑区域 进行性核上性麻痹:额叶和中脑区域 |
| [70] | 肌萎缩侧索硬化 症(14) | 57±12 | 头部摇篮/ SE-EPI | 2×2×2 | TI | C33: 27.63±2.05 (50 Hz), 40.39±3.68 (60 Hz); C44: 4.21±0.06 (50 Hz), 4.68±0.07 (60 Hz); C66: 4.89±0.09 (50 Hz), 5.35±0.13 (60 Hz) | 皮质脊髓束 |
| [71] | 行为性额颞叶痴呆 (5) | 55~66 | 气动枕/ SE-EPI | 3×3×3 | DI | 对照组:2.77(中位数) 疾病组:2.57(中位数) | 额叶和颞叶 |
| [72] | 阿尔茨海默症(8) 路易体痴呆(5) 额颞叶痴呆(5) 常压脑积水(20) | 78~87 63~76 54~65 60~86 | 气动枕/ SE-EPI | 3×3×3 | DI | 对照组:2.44±0.08 阿尔茨海默症:2.32±0.09 路易体痴呆:2.43±0.11 额颞叶痴呆:2.28±0.10 常压脑积水:2.46 ±0.08 | 阿尔茨海默症:额叶、颞叶、顶叶和运动感知区 路易体痴呆:无 额颞叶痴呆:额叶和颞叶 常压脑积水:顶叶、枕叶和运动感知区 |
*测量的结果中,CN-表示PET检验Aβ阴性,认知正常的样本;CN+表示PET检验Aβ阳性,认知正常的样本;C33、C44、C66表示线弹性材料的6×6硬度矩阵中,对角线上从上到下第3、4、6个参量.其余数据为剪切模量幅值.
在AD的研究方面,美国梅奥医学中心的Murphy等人最早使用MRE对AD患者开展了研究[64](表2).其研究发现AD患者的脑组织剪切模量幅值相较于正常对照组有显著下降.在随后的研究中,Murphy等人进一步与PET诊断结果进行对比以验证其结论,并发现剪切模量幅值下降的区域主要分布在额叶、颞叶和顶叶[65]. 梅奥中心的团队在近期的研究中还发现剪切模量在中颞叶部分也会显著下降[66].Gerischer等人也从全脑角度发现剪切模量幅值在AD患者中显著下降,并发现海马体的剪切模量幅角
在PD方面,德国Charite的Lipp等人最早针对PD和进行性核上性麻痹(progressive supranuclear palsy,PSP)开展了研究,发现PD患者的全脑的剪切模量幅值对比正常人有显著下降,尤其是在额叶和中脑区[69].对于PSP患者,其脑组织剪切模量的幅角也有显著下降,但与PD患者的下降幅度有明显差异.另外,基于轴向同性的模量估计,Romano等人发现肌萎缩侧索硬化症(amyotrophic lateral sclerosis,ALS)患者的相关轴向同性模量分量有显著下降[70].而针对行为性额颞叶痴呆(behavioral variant fronto-temporal dementia,bvFTD)的MRE测量则发现了相关患者在全脑、额叶和颞叶区域剪切模量幅值下降的特点[71].ElSheikh等人在2017年前开展过一系列有关神经退行性疾病的研究[72].其结果中,路易体痴呆(Lewy bodies,DLB)患者没有发现显著的剪切模量幅值变化,而对于有组织剪切模量幅值下降的疾病,AD的下降区域主要集中在额叶、颞叶、顶叶和运动区;额颞叶痴呆(FTD)主要集中在额叶和颞叶;常压脑积水(normal pressure hydrocephalus,NPH)的主要集中在枕叶、顶叶和运动区.梅奥医学中心的Pavuluri 等人也发现FTD患者的额叶、内侧颞叶有显著的剪切模量幅值下降[66].
3 MRE在脑疾病研究中的前沿与趋势
本节从技术方法和临床应用两方面,基于近期在脑MRE的研究进展,对现有研究前沿开展讨论,并对未来脑MRE的研究和应用做趋势判断.
3.1 技术方法
成像速度的提升一直是磁共振成像所追求的目标.由于现有MRE成像序列主要通过在现有的常规序列上添加位移编码梯度实现,其代价是MRE的成像时间也受到基础序列扫描时间的限制,一般最常用的全脑单频率3 mm各向同性像素MRE的成像时间不会低于5 min.在MRE技术本身和MRE在脑组织的应用方面,提高成像速率是当前提升MRE扫描效率的重要方向.McIlvain等人将MRE图像用可分离函数进行时空分解[73⇓-75],并通过采集导航图像找到时间基,同时基于MRE图像的低秩特性,对在k空间欠采样的图像进行重建[76].他们所提出的OCILLATE方法可实现全脑2 mm各向同性像素MRE成像,总时间1 min 48 s.梅奥医学中心的Peng等人提出了使用梯度自旋回波层叠螺旋采样的方式开展3D全脑MRE[77]的构想.通过结合压缩感知方法的重建,以及基于结构像的去模糊算法,最总实现了5 min内全脑2 mm各向同性像素MRE成像.梅奥医学中心Sui等人则提出了一种类似螺旋桨成像的,基于SE-EPI的径向采集方式TURBINE-MRE[78].在k空间中,EPI的轨迹在径向方向旋转,从而对目标信号进行3D采集.运用这种方法可实现5 min内全脑1.6 mm各向同性像素MRE成像.上海交大Wang等人基于空间堆叠径向采集方式,将每次k空间记录的径向采样分配到周期波动的相位中以实现采集加速[19].利用波动图像在时域中的稀疏性,通过结合时间和空间域中的稀疏约束的压缩感知实现采样加速,最终在联合采集加速后将全脑MRE成像速度加速了20倍,从而实现了2 min全脑3 mm各向同性像素MRE成像,而传统的GRE-MRE成像则需要至少40 min.由于MRE记录的是周期波动位移,因此其时间和空间域的可压缩性有很大利用空间.同时,随着快速成像技术的发展,MRE的全脑3D成像速度还有许多可以继续提升的空间.
在一次采集中实现多种成像功能的多模态成像,是当前脑成像的研究热点之一.基于MRE的多模态脑成像,同样也是当前MRE成像技术发展的主要方向之一.斯坦福大学的Lan等人基于SE序列,将位移编码梯度与功能磁共振成像(functional MRI,fMRI)序列相结合,开发了fMRI/fMRE序列,实现了单次激发后两种模态的同时采集[79].其中幅值图呈现fMRI的信号,相位图呈现MRE的信号.美国伊利诺伊大学的Yin等人通过将扩散张量成像(diffusion tensor imaging,DTI)的扩散梯度和MRE中的位移编码梯度组合排列,实现了基于幅值图的扩散记录和基于幅角图的位移记录[80].由于MRE是基于氢原子空间相位积累的成像,只利用了所采集图像的幅角信息.因此,采集的幅值图像理论上可以通过前期设计集成其他功能成像的原理以实现多模态采集.可以预见,MRE成像将会与其他的脑成像方法相结合,提供更丰富的多模态信息.
无驱动器的脑组织MRE成像是采用人体自身的血流搏动所产生的微小脑组织位移开展的测量.基于非谐振的组织生物力学参量的反算,主要基于尾波的相关计算开展[81],通过对互不相干波动的混合相关分析开展组织的力学参量测量.脑组织的无驱动测量已有前期研究[82],但囿于MRE的成像时间,其能捕捉的波动难以达到很高时间分辨率.同时,脑组织内血流搏动产生的微小位移对MRE的采集信噪比提出了很高要求,而现阶段的MRE序列在信噪比方面难有较大提升.这些都限制了基于尾波的反演计算精度.但是,由于不需要额外的振动硬件,无驱动的脑组织MRE具有显著的临床应用价值,如以上技术问题得以解决将为未来MRE技术的普及提供更坚实的助力.
现有针对脑组织的MRE测量,主要都基于组织各向同性的假设[15,34,38,83].已有研究表明,人脑灰质显示出较好的各向同性力学特性[84],但是人脑白质本身具有显著的纤维方向,其力学特性在沿纤维方向和垂直纤维方向具有显著差异[84⇓-86].虽然各向同性的本构假设可以满足脑肿瘤和脑积水等病变的研究[35,38,87],但是针对脑白质的研究如果依然采用各向同性本构假设则难以对其生物力学参数进行准确测量.美国伊利诺伊大学香槟分校的Gerischer等人通过MRE方法测量了剪切波动在前-后和左-右两个不同方向传播状态下脑白质的剪切模量,发现在不同传播方向下,脑白质的剪切模量相差可达33%[67].针对脑白质纤维加固的特征,采用横观各向同性模型进行建模是主要的方法.飞利浦研究实验室的Sinkus等人最早基于横观各向同性模型分析剪切波动的传播,并针对于乳腺生物力学参数的测量提出了相关参数的计算方法[88].美国海军研究所Romano等人利用DTI确定脑白质纤维方向后,结合Helmholtz分解将滤波后的波动位移用横观各向同性的一般方程进行逆向求解,并最终得到了模型所需的5个参数的计算结果[89].近年来,基于横观各向同性的组织模量反演算法已成为热点.美国达特茅斯学院的Mcgarry等人使用DTI获得神经纤维的方向后,通过建立轴向同性的脑组织模型和结合有限元反算的方法,取得了较好的反演效果[90].其团队近期还将横观各向同性的模型扩展到衰减参量中[91].欧洲的Fovargue等人和澳洲Babaei等人合作,也提出了基于横观各向同性的有限元反演算法[92].美国俄亥俄大学的Kalra等人通过对不同方向的波动开展空间和频率的滤波,并结合简化的各向异性波动方程,对各向异性的本构方程中弹性矩阵的各个元素开展求解[93].美国圣路易斯华盛顿大学的Hou等人利用神经网络对不可压缩三参数横观各向同性模型开展了反演[94].综上所述,基于脑组织的生理结构进一步提升MRE测量的脑组织生物力学参量的准确性,将成为MRE领域另一个研究热点.
3.2 临床应用
临床应用的操作便捷性和患者舒适性一直是磁共振新技术方法临床转化时的重要考虑因素.MRE需要使用额外的振动装置,因此在患者的扫描摆位、技术员的操作流程等方面相比常规扫描多了一些工序,一定程度上降低了操作的效率.因此,在无驱动器的MRE方面,一直有研究者期望通过其他参数指标来对组织的生物力学参量开展间接测量.法国的Le Bihan等人最早提出采用扩散成像的方法,对组织的模量开展间接计算的虚拟MRE方法(virtual MRE,vMRE)[95].此方法应用高低b值的扩散成像数据,通过计算对组织模量开展估计,并最早在肝脏开展了应用验证[96].在脑组织的应用方面,瑞典的Lagerstrand等人采用vMRE对垂体瘤开展了临床研究[97].
虽然现阶段除了肿瘤和退行性疾病外,MRE在脑疾病的临床应用中尚不多见.但在原理上,只要组织的力学特性参量与疾病的发生和进展相关联,即可应用MRE开展诊断与预后的探索.因此,MRE在脑疾病和脑科学中的应用具有广阔前景.
4 总结与展望
利益冲突
无
参考文献
Effects of extracellular matrix viscoelasticity on cellular behaviour
[J].
Fifty shades of brain: a review on the mechanical testing and modeling of brain tissue
[J].
Perspective: Challenges and opportunities in computational brain mechanics research: How can we use recent experimental data to improve models of brain mechanics?
[J].
Viscoelastic response of neural cells governed by the deposition of amyloid-β peptides (Aβ)
[J].
Solid stress and elastic energy as measures of tumour mechanopathology
[J].
REVIEW: MR elastography of brain tumors
[J].
Magnetic resonance elastography from fundamental soft-tissue mechanics to diagnostic imaging
[J].
MR elastography: Principles, guidelines, and terminology
[J].
DOI:10.1002/mrm.28627
PMID:33296103
[本文引用: 2]

Magnetic resonance elastography (MRE) is a phase contrast-based MRI technique that can measure displacement due to propagating mechanical waves, from which material properties such as shear modulus can be calculated. Magnetic resonance elastography can be thought of as quantitative, noninvasive palpation. It is increasing in clinical importance, has become widespread in the diagnosis and staging of liver fibrosis, and additional clinical applications are being explored. However, publications have reported MRE results using many different parameters, acquisition techniques, processing methods, and varied nomenclature. The diversity of terminology can lead to confusion (particularly among clinicians) about the meaning of and interpretation of MRE results. This paper was written by the MRE Guidelines Committee, a group formalized at the first meeting of the ISMRM MRE Study Group, to clarify and move toward standardization of MRE nomenclature. The purpose of this paper is to (1) explain MRE terminology and concepts to those not familiar with them, (2) define "good practices" for practitioners of MRE, and (3) identify opportunities to standardize terminology, to avoid confusion.© 2020 International Society for Magnetic Resonance in Medicine.
A multi-purpose electromagnetic actuator for magnetic resonance elastography
[J].
DOI:S0730-725X(18)30057-2
PMID:29679635
[本文引用: 1]

An electromagnetic actuator was designed for magnetic resonance elastography (MRE). The actuator is unique in that it is simple, portable, and capable of brain, abdomen, and phantom imagings. A custom-built control unit was used for controlling the vibration frequency and synchronizing the trigger signals. An actuation unit was built and mounted on the specifically designed clamp and holders for different imaging applications. MRE experiments with respect to gel phantoms, brain, and liver showed that the actuator could produce stable and consistent mechanical waves. Estimated shear modulus using local frequency estimate method demonstrated that the measurement results were in line with that from MRE studies using different actuation systems. The relatively easy setup procedure and simple design indicated that the actuator system had the potential to be applied in many different clinical studies.Copyright © 2018 Elsevier Inc. All rights reserved.
A longitudinal magnetic resonance elastography study of murine brain tumors following radiation therapy
[J].
Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations
[J].
DOI:10.1148/radiol.2015142141
PMID:26162026
[本文引用: 1]

To assess the technical success rate and diagnostic performance of liver magnetic resonance (MR) elastography.This retrospective study was approved by the institutional review board with patient informed consent. A total of 1377 consecutive MR elastography examinations performed between 2007 and 2010 in 1287 patients for clinical indications were included. Medical records were used to retrieve liver stiffness as assessed with MR elastography, histologic analysis, blood work, and other liver disease-related information. Nonparametric Kruskal-Wallis tests and analysis of covariance methods were used to evaluate the diagnostic values and relationships of the collected data.Hepatic MR elastography had a success rate of 94.4% (1300 of 1377 cases) and yielded reproducible measurements (r = 0.9716, P <.0001) in the study cohort, with a complex patient profile and multiple interpreters. Body mass index had no significant effect on success rate (P =.2). In 289 patients who underwent liver biopsy within 1 year of the MR elastography date, mean liver stiffness as assessed with MR elastography was significantly higher in patients with advanced fibrosis (stages F3, F4) than in those with mild to moderate fibrosis (stages F0, F1, F2) (5.93 kPa ± 2.31 [standard deviation] vs 3.35 kPa ± 1.44, P <.0001). Liver stiffness is associated with many factors other than fibrosis extent, including cause of fibrosis (viral hepatitis C vs nonalcoholic fatty liver disease, P =.025), inflammation (severe vs mild to moderate, P =.03), and hepatic metabolic and synthetic function (no fibrosis vs intermediate fibrosis, P ≤.01).In a general clinical practice environment, hepatic MR elastography is a robust imaging method with a high success rate in a broad spectrum of patients. It also shows the complex association between liver stiffness and hepatic pathophysiology.© RSNA, 2015 Online supplemental material is available for this article.
Diffusion tensor magnetic resonance imaging for predicting the consistency of intracranial meningiomas
[J].
2D approximation of 3D wave propagation in MR elastography of the brain
[C]//
Harnessing brain waves: a review of brain magnetic resonance elastography for clinicians and scientists entering the field
[J].
A flow velocity zeugmatographic interlace for NMR imaging in humans
[J].We describe a flow sensitizing zeugmatographic phase-modulation interlace for NMR-imaging which is exactly analogous to Lauterbur's spatial-location-sensitizing magnetic field gradients. The method may be implemented by minor modification of any NMR-imaging scanner without interfering with its conventional operation, and enables up to 6-D imaging of the joint (spatial-flow) density of spins delta (r,v). In a special simplification, specific-flow-density, mean value of v(r), and flow-current-specific-flow-density, rho 0(r)v, derive directly from "real' and "imaginary" parts of the image reconstruction.
Prostate MR elastography with transperineal electromagnetic actuation and a fast fractionally encoded steady-state gradient echo sequence
[J].
Analysis and improvement of motion encoding in magnetic resonance elastography
[J].
Fast magnetic resonance elastography with multiphase radial encoding and harmonic motion sparsity based reconstruction
[J].
Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation
[J].Noninvasive quantitation of the mechanical properties of tissue could improve early detection of pathology. Previously a method for detecting displacement from propagating shear waves using a phase-contrast MRI technique was developed. In this work it is demonstrated how a collection of data representing the full vector displacement field could be used to potentially estimate the full complex stiffness tensor. An algebraic inversion approach useful for piece-wise homogeneous materials is described in detail for the general isotropic case, which is then specialized to incompressible materials as a model for tissue. Results of the inversion approach are presented for simulated and experimental phantom data that show the technique can be used to obtain shear wave-speed and attenuation in regions where there is sufficient signal-to-noise ratio in the displacement and its second spatial derivatives. The sensitivity to noise is higher in the attenuation estimates than the shear wave-speed estimates. Magn Reson Med 45:299-310, 2001.Copyright 2001 Wiley-Liss, Inc.
On the noninvasive determination of material parameters from a knowledge of elastic displacements theory and numerical simulation
[J].
Algebraic Helmholtz inversion in planar magnetic resonance elastography
[J].
Viscoelastic shear properties of in vivo breast lesions measured by MR elastography
[J].
DOI:10.1016/j.mri.2004.11.060
PMID:15833607
[本文引用: 1]

Elastography is a technique to assess the viscoelastic properties of tissue by measuring an acoustic wave propagating though the object. Here, the technique is applied in the course of standard MR mammography to 15 patients with different pathologies (six breast cancer cases, six fibroadenoma cases and three mastopathy cases). Low-frequency mechanical waves are coupled longitudinally into the tissue in order to obtain sufficient wave amplitude throughout the entire breast. This leads to the presence of a substantial fraction of compressional waves, which contribute to the total displacement field. It is shown theoretically that the correct evaluation of these contributions from the compressional wave is rather difficult due to the almost incompressible nature of tissue. To overcome this problem, it is proposed to apply the curl-operator to the measured displacement field in order to completely remove contributions from the compressional wave. Results from simulations and a breast phantom demonstrate the feasibility of the technique. The in vivo results show a good separation between breast cancer and benign fibroadenoma utilizing the shear modulus. Breast cancer appears on average 2.2 (P<.001) times stiffer. All breast cancer cases showed a good delineation to the surrounding breast tissue with an average elevation of a factor of 3.3 (P< 1.4 x 10(-6)). The results as obtained for the shear viscosity do not indicate to be useful for separating benign from malignant lesions.
An overlapping subzone technique for MR-based elastic property reconstruction
[J].
DOI:10.1002/(sici)1522-2594(199910)42:4<779::aid-mrm21>3.0.co;2-z
PMID:10502768
[本文引用: 1]

A finite element-based nonlinear inversion scheme for magnetic resonance (MR) elastography is detailed. The algorithm operates on small overlapping subzones of the total region of interest, processed in a hierarchical order as determined by progressive error minimization. This zoned approach allows for a high degree of spatial discretization, taking advantage of the data-rich environment afforded by the MR. The inversion technique is tested in simulation under high-noise conditions (15% random noise applied to the displacement data) with both complicated user-defined stiffness distributions and realistic tissue geometries obtained by thresholding MR image slices. In both cases the process has proved successful and has been capable of discerning small inclusions near 4 mm in diameter. Magn Reson Med 42:779-786, 1999.Copyright 1999 Wiley-Liss, Inc.
Magnetic resonance elastography: Non-invasive mapping of tissue elasticity
[J].Magnetic resonance elastography (MRE) is a phase-contrast-based MRI imaging technique that can directly visualize and quantitatively measure propagating acoustic strain waves in tissue-like materials subjected to harmonic mechanical excitation. The data acquired allows the calculation of local quantitative values of shear modulus and the generation of images that depict tissue elasticity or stiffness. This is significant because palpation, a physical examination that assesses the stiffness of tissue, can be an effective method of detecting tumors, but is restricted to parts of the body that are accessible to the physician's hand. MRE shows promise as a potential technique for 'palpation by imaging', with possible applications in tumor detection (particularly in breast, liver, kidney and prostate), characterization of disease, and assessment of rehabilitation (particularly in muscle). We describe MRE in the context of other recent techniques for imaging elasticity, discuss the processing algorithms for elasticity reconstruction and the issues and assumptions they involve, and present recent ex vivo and in vivo results.
Local multiscale frequency and bandwidth estimation
[C]//
Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves
[J].
DOI:S1361-8415(16)00002-5
PMID:26845371
[本文引用: 1]

Palpation is one of the most sensitive, effective diagnostic practices, motivating the quantitative and spatially resolved determination of soft tissue elasticity parameters by medical ultrasound or MRI. However, this so-called elastography often suffers from limited anatomical resolution due to noise and insufficient elastic deformation, currently precluding its use as a tomographic modality on its own. We here introduce an efficient way of processing wave images acquired by multifrequency magnetic resonance elastography (MMRE), which relies on wave number reconstruction at different harmonic frequencies followed by their amplitude-weighted averaging prior to inversion. This results in compound maps of wave speed, which reveal variations in tissue elasticity in a tomographic fashion, i.e. an unmasked, slice-wise display of anatomical details at pixel-wise resolution. The method is demonstrated using MMRE data from the literature including abdominal and pelvic organs such as the liver, spleen, uterus body and uterus cervix. Even in small regions with low wave amplitudes, such as nucleus pulposus and spinal cord, elastic parameters consistent with literature values were obtained. Overall, the proposed method provides a simple and noise-robust strategy of in-plane wave analysis of MMRE data, with a pixel-wise resolution producing superior detail to MRE direct inversion methods. Copyright © 2016 Elsevier B.V. All rights reserved.
A heterogenous, time harmonic, nearly incompressible transverse isotropic finite element brain simulation platform for MR elastography
[J].
Shear wave propagation and estimation of material parameters in a nonlinear, fibrous material
[J].
Magnetic resonance elastography of slow and fast shear waves illuminates differences in shear and tensile moduli in anisotropic tissue
[J].
DOI:S0021-9290(16)30159-2
PMID:26920505
[本文引用: 1]

Mechanical anisotropy is an important property of fibrous tissues; for example, the anisotropic mechanical properties of brain white matter may play a key role in the mechanics of traumatic brain injury (TBI). The simplest anisotropic material model for small deformations of soft tissue is a nearly incompressible, transversely isotropic (ITI) material characterized by three parameters: minimum shear modulus (µ), shear anisotropy (ϕ=µ1µ-1) and tensile anisotropy (ζ=E1E2-1). These parameters can be determined using magnetic resonance elastography (MRE) to visualize shear waves, if the angle between the shear-wave propagation direction and fiber direction is known. Most MRE studies assume isotropic material models with a single shear (µ) or tensile (E) modulus. In this study, two types of shear waves, "fast" and "slow", were analyzed for a given propagation direction to estimate anisotropic parameters µ, ϕ, and ζ in two fibrous soft materials: turkey breast ex vivo and aligned fibrin gels. As expected, the speed of slow shear waves depended on the angle between fiber direction and propagation direction. Fast shear waves were observed when the deformations due to wave motion induced stretch in the fiber direction. Finally, MRE estimates of anisotropic mechanical properties in turkey breast were compared to estimates from direct mechanical tests.Copyright © 2016 Elsevier Ltd. All rights reserved.
High-resolution magnetic resonance elastography reveals differences in subcortical gray matter viscoelasticity between young and healthy older adults
[J].
DOI:S0197-4580(18)30018-6
PMID:29494862
[本文引用: 2]

Volumetric structural magnetic resonance imaging (MRI) is commonly used to determine the extent of neuronal loss in aging, indicated by cerebral atrophy. The brain, however, exhibits other biophysical characteristics such as mechanical properties, which can be quantified with magnetic resonance elastography (MRE). MRE is an emerging noninvasive imaging technique for measuring viscoelastic tissue properties, proven to be sensitive metrics of neural tissue integrity, as described by shear stiffness, μ and damping ratio, ξ parameters. The study objective was to evaluate global and regional MRE parameter differences between young (19-30 years, n = 12) and healthy older adults (66-73 years, n = 12) and to assess whether MRE measures provide additive value over volumetric magnetic resonance imaging measurements. We investigated the viscoelasticity of the global cerebrum and 6 regions of interest (ROIs) including the amygdala, hippocampus, caudate, pallidum, putamen, and thalamus. In older adults, we found a decrease in μ in all ROIs, except for the hippocampus, indicating widespread brain softening; an effect that remained significant after controlling for ROI volume. In contrast, the relative viscous-to-elastic behavior of the brain ξ did not differ between age groups, suggesting a preservation of the organization of the tissue microstructure. These data support the use of MRE as a novel imaging biomarker for characterizing age-related differences to neural tissue not captured by volumetric imaging alone.Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Use of a Rayleigh damping model in elastography
[J].
DOI:10.1007/s11517-008-0356-5
PMID:18521645
[本文引用: 1]

A Rayleigh damping model applied to magnetic resonance elastography incorporates attenuation behavior proportionally related to both elastic and inertial forces, and allows two damping parameters to be extracted from an MRI motion dataset. Under time-harmonic conditions, the model can be implemented by the use of complex shear modulus and density, whereas viscoelastic damping models commonly used in elastography consist of only a complex shear modulus, and model only a single damping effect. Simulation studies reveal that the differences between damped elastic behavior resulting from a purely complex shear modulus (CSM damping) and from a purely complex density (CD damping) become larger as the overall level of damping present (indicated by the damping ratio) increases. A plot of results generated from the finite element (FE) model indicate the relative motion differences estimated for a range of damping ratios and CSM/CD damping combinations increase with damping ratio, and can be up to 15% at a damping ratio of 50% and therefore using the correct model for a Rayleigh damped material becomes increasingly important as damping levels increase. Resonance-related effects cause values from this plot to vary by as much as 3% as parameters such as wave speed, frequency, and problem size are altered. These motion differences can be compared to expected noise levels to estimate the parameter resolution achievable by a reconstruction algorithm. An optimization-based global property reconstruction algorithm was developed, and used for testing Rayleigh damping parameter reconstructions with gaussian noise added to the simulated motion input data. The coherent motion errors resulting from altering the combination of the two damping parameters are large enough to allow accurate determination of both of the Rayleigh damping parameters with incoherent noise levels comparable to MR measurements. The accuracy achieved by the global reconstructions was significantly better than would be predicted by examining the motion differences for differing CSM/CD damping combinations, which is likely to be due to the low ratio between number of reconstructed parameters and number of noisy measurements.
Impact of material homogeneity assumption on cortical stiffness estimates by MR elastography
[J].
DOI:10.1002/mrm.29226
PMID:35381121
[本文引用: 1]

Inversion algorithms used to convert acquired MR elastography wave data into material property estimates often assume that the underlying materials are locally homogeneous. Here we evaluate the impact of that assumption on stiffness estimates in gray-matter regions of interest in brain MR elastography.We describe an updated neural network inversion framework using finite-difference model-derived data to train convolutional neural network inversion algorithms. Neural network inversions trained on homogeneous simulations (homogeneous learned inversions [HLIs]) or inhomogeneous simulations (inhomogeneous learned inversions [ILIs]) are generated with a variety of kernel sizes. These inversions are evaluated in a brain MR elastography simulation experiment and in vivo in a test-retest repeatability experiment including 10 healthy volunteers.In simulation and in vivo, HLI and ILI with small kernels produce similar results. As kernel size increases, the assumption of homogeneity has a larger effect, and HLI and ILI stiffness estimates show larger differences. At each inversion's optimal kernel size in simulation (7 × 7 × 7 for HLI, 11 × 11 × 11 for ILI), ILI is more sensitive to true changes in stiffness in gray-matter regions of interest in simulation. In vivo, there is no difference in the region-level repeatability of stiffness estimates between the inversions, although ILI appears to better maintain the stiffness map structure as kernel size increases, while decreasing the spatial variance in stiffness estimates.This study suggests that inhomogeneous inversions provide small but significant benefits even when large stiffness gradients are absent.© 2022 International Society for Magnetic Resonance in Medicine.
An electromagnetic actuator for brain magnetic resonance elastography with high frequency accuracy
[J].
How tissue fluidity influences brain tumor progression
[J].
Paediatric brain tissue properties measured with magnetic resonance elastography
[J].
Robustness of MR elastography in the healthy brain: repeatability, reliability, and effect of different reconstruction methods
[J].
DOI:10.1002/jmri.27475
PMID:33403750
[本文引用: 1]

Changes in brain stiffness can be an important biomarker for neurological disease. Magnetic resonance elastography (MRE) quantifies tissue stiffness, but the results vary between acquisition and reconstruction methods.To measure MRE repeatability and estimate the effect of different reconstruction methods and varying data quality on estimated brain stiffness.Prospective.Fifteen healthy subjects.3T MRI, gradient-echo elastography sequence with a 50 Hz vibration frequency.Imaging was performed twice in each subject. Images were reconstructed using a curl-based and a finite-element-model (FEM)-based method. Stiffness was measured in the whole brain, in white matter, and in four cortical and four deep gray matter regions. Repeatability coefficients (RC), intraclass correlation coefficients (ICC), and coefficients of variation (CV) were calculated. MRE data quality was quantified by the ratio between shear waves and compressional waves.Median values with range are presented. Reconstruction methods were compared using paired Wilcoxon signed-rank tests, and Spearman's rank correlation was calculated between MRE data quality and stiffness. Holm-Bonferroni corrections were employed to adjust for multiple comparisons.In the whole brain, CV was 4.3% and 3.8% for the curl and the FEM reconstruction, respectively, with 4.0-12.8% for subregions. Whole-brain ICC was 0.60-0.74, ranging from 0.20 to 0.89 in different regions. RC for the whole brain was 0.14 kPa and 0.17 kPa for the curl and FEM methods, respectively. FEM reconstruction resulted in 39% higher stiffness than the curl reconstruction (P < 0.05). MRE data quality, defined as shear-compression wave ratio, was higher in peripheral regions than in central regions of the brain (P < 0.05). No significant correlations were observed between MRE data quality and stiffness estimates.MRE of the human brain is a robust technique in terms of repeatability. Caution is warranted when comparing stiffness values obtained with different techniques.1 TECHNICAL EFFICACY STAGE: 1.© 2021 The Authors. Journal of Magnetic Resonance Imaging published by Wiley Periodicals LLC. on behalf of International Society for Magnetic Resonance in Medicine.
A new method for quantification and 3D visualization of brain tumor adhesion using slip interface imaging in patients with meningiomas
[J].
DOI:10.1007/s00330-021-07918-6
PMID:33852045
[本文引用: 3]

To develop an objective quantitative method to characterize and visualize meningioma-brain adhesion using MR elastography (MRE)-based slip interface imaging (SII).This retrospective study included 47 meningiomas (training dataset: n = 35; testing dataset: n = 12) with MRE/SII examinations. Normalized octahedral shear strain (NOSS) values were calculated from the acquired MRE displacement data. The change in NOSS at the tumor boundary (ΔNOSS) was computed, from which a 3D ΔNOSS map of the tumor surface was created and the probability distribution of ΔNOSS over the entire tumor surface was calculated. Statistical features were calculated from the probability histogram. After eliminating highly correlated features, the capability of the remaining feature for tumor adhesion classification was assessed using a one-way ANOVA and ROC analysis.The magnitude and location of the tumor adhesion can be visualized by the reconstructed 3D ΔNOSS surface map. The entropy of the ΔNOSS histogram was significantly different between adherent tumors and partially/completely non-adherent tumors in both the training (AUC: 0.971) and testing datasets (AUC: 0.900). Based on the cutoff values obtained from the training set, the ΔNOSS entropy in the testing dataset yielded an accuracy of 0.83 for distinguishing adherent versus partially/non-adherent tumors, and 0.67 for distinguishing non-adherent versus completely/partially adherent tumors.SII-derived ΔNOSS values are useful for quantification and classification of meningioma-brain adhesion. The reconstructed 3D ΔNOSS surface map presents the state and location of tumor adhesion in a "clinician-friendly" manner, and can identify meningiomas with a high risk of adhesion to adjacent brain parenchyma.• MR elastography (MRE)-based slip interface imaging shows promise as an objective tool to preoperatively discriminate meningiomas with a high risk of intraoperative adhesion. • Measurement of the change of shear strain at meningioma boundaries can provide quantitative metrics depicting the state of adhesion at the tumor-brain interface. • The surface map of tumor adhesion shows promise in assisting precise adhesion localization, using a comprehensible, "clinician-friendly" 3D visualization.
Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography
[J].
DOI:10.1002/jmri.22707
PMID:21751286
[本文引用: 1]

To test patient acceptance and reproducibility of the 3D magnetic resonance elastography (MRE) brain exam using a soft vibration source, and to determine if MRE could noninvasively measure a change in the elastic properties of the brain parenchyma due to Alzheimer's disease (AD).MRE exams were performed using an accelerated spin-echo echo planar imaging (EPI) pulse sequence and stiffness was calculated with a 3D direct inversion algorithm. Reproducibility of the technique was assessed in 10 male volunteers, who each underwent four MRE exams separated into two imaging sessions. The effect of AD on brain stiffness was assessed in 28 volunteers, 7 with probable AD, 14 age- and gender-matched PIB-negative (Pittsburgh Compound B, a PET amyloid imaging ligand) cognitively normal controls (CN-), and 7 age- and gender-matched PIB-positive cognitively normal controls (CN+).The median stiffness of the 10 volunteers was 3.07 kPa with a range of 0.40 kPa. The median and maximum coefficients of variation for these volunteers were 1.71% and 3.07%. The median stiffness of the 14 CN- subjects was 2.37 kPa (0.44 kPa range) compared to 2.32 kPa (0.49 kPa range) within the CN+ group and 2.20 kPa (0.33 kPa range) within the AD group. A significant difference was found between the three groups (P = 0.0055, Kruskal-Wallis one-way analysis of variance). Both the CN+ and CN- groups were significantly different from the AD group.3D MRE of the brain can be performed reproducibly and demonstrates significantly reduced brain tissue stiffness in patients with AD.Copyright © 2011 Wiley-Liss, Inc.
Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography
[J].
DOI:10.1002/nbm.1189
PMID:17614101
[本文引用: 1]

The purpose of this work was to develop magnetic resonance elastography (MRE) for the fast and reproducible measurement of spatially averaged viscoelastic constants of living human brain. The technique was based on a phase-sensitive echo planar imaging acquisition. Motion encoding was orthogonal to the image plane and synchronized to intracranial shear vibrations at driving frequencies of 25 and 50 Hz induced by a head-rocker actuator. Ten time-resolved phase-difference wave images were recorded within 60 s and analyzed for shear stiffness and shear viscosity. Six healthy volunteers (six men; mean age 34.5 years; age range 25-44 years) underwent 23-39 follow-up MRE studies over a period of 6 months. Interindividual mean +/- SD shear moduli and shear viscosities were found to be 1.17 +/- 0.03 kPa and 3.1 +/- 0.4 Pas for 25 Hz and 1.56 +/- 0.07 kPa and 3.4 +/- 0.2 Pas for 50 Hz, respectively (P < or = 0.01). The intraindividual range of shear modulus data was 1.01-1.31 kPa (25 Hz) and 1.33-1.77 kPa (50 Hz). The observed modulus dispersion indicates a limited applicability of Voigt's model to explain viscoelastic behavior of brain parenchyma within the applied frequency range. The narrow distribution of data within small confidence intervals demonstrates excellent reproducibility of the experimental protocol. The results are necessary as reference data for future comparisons between healthy and pathological human brain viscoelastic data.Copyright (c) 2007 John Wiley & Sons, Ltd.
Cerebral multifrequency MR elastography by remote excitation of intracranial shear waves
[J].
DOI:10.1002/nbm.3388
PMID:26373228
[本文引用: 1]

The aim of this study was to introduce remote wave excitation for high-resolution cerebral multifrequency MR elastography (mMRE). mMRE of 25-45-Hz drive frequencies by head rocker stimulation was compared with mMRE by remote wave excitation based on a thorax mat in 12 healthy volunteers. Maps of the magnitude |G*| and phase φ of the complex shear modulus were reconstructed using multifrequency dual elasto-visco (MDEV) inversion. After the scan, the subjects and three operators assessed the comfort and convenience of cerebral mMRE using two methods of stimulating the brain. Images were acquired in a coronal view in order to identify anatomical regions along the spinothalamic pathway. In mMRE by remote actuation, all subjects and operators appreciated an increased comfort and simplified procedural set-up. The resulting strain amplitudes in the brain were sufficiently large to analyze using MDEV inversion, and yielded high-resolution viscoelasticity maps which revealed specific anatomical details of brain mechanical properties: |G*| was lowest in the pons (0.97 ± 0.08 kPa) and decreased within the corticospinal tract in the caudal-cranial direction from the crus cerebri (1.64 ± 0.26 kPa) to the capsula interna (1.29 ± 0.14 kPa). By avoiding onerous mechanical stimulation of the head, remote excitation of intracranial shear waves can be used to measure viscoelastic parameters of the brain with high spatial resolution. Therewith, the new mMRE method is suitable for neuroradiological examinations in the clinic.Copyright © 2015 John Wiley & Sons, Ltd.
Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications
[J].
Magnetic resonance elastography in the study of neurodegenerative diseases
[J].
Local mechanical properties of white matter structures in the human brain
[J].
DOI:10.1016/j.neuroimage.2013.04.089
PMID:23644001
[本文引用: 1]

The noninvasive measurement of the mechanical properties of brain tissue using magnetic resonance elastography (MRE) has emerged as a promising method for investigating neurological disorders. To date, brain MRE investigations have been limited to reporting global mechanical properties, though quantification of the stiffness of specific structures in the white matter architecture may be valuable in assessing the localized effects of disease. This paper reports the mechanical properties of the corpus callosum and corona radiata measured in healthy volunteers using MRE and atlas-based segmentation. Both structures were found to be significantly stiffer than overall white matter, with the corpus callosum exhibiting greater stiffness and less viscous damping than the corona radiata. Reliability of both local and global measures was assessed through repeated experiments, and the coefficient of variation for each measure was less than 10%. Mechanical properties within the corpus callosum and corona radiata demonstrated correlations with measures from diffusion tensor imaging pertaining to axonal microstructure.Copyright © 2013 Elsevier Inc. All rights reserved.
MR elastography frequency-dependent and independent parameters demonstrate accelerated decrease of brain stiffness in elder subjects
[J].
Magnetic resonance elastography of brain tumors: Preliminary results
[J].To investigate the potential value of magnetic resonance elastography (MRE) in evaluating the consistency of brain tumors.Six patients with known solid brain tumor underwent brain MRE studies. Consistency of brain tumors was evaluated at surgery. Correspondence of MRE evaluation with operative result was studied.The elasticity of tumors in six patients evaluated by MRE agreed with the tumor consistency given by the operative results.MRE could be used as an imaging technique for noninvasive assessment of the consistency of brain tumor in vivo.
Preoperative assessment of meningioma stiffness using magnetic resonance elastography
[J].
DOI:10.3171/2012.9.JNS12519
PMID:23082888
[本文引用: 2]

The object of this study was to determine the potential of magnetic resonance elastography (MRE) to preoperatively assess the stiffness of meningiomas.Thirteen patients with meningiomas underwent 3D brain MRE examination to measure stiffness in the tumor as well as in surrounding brain tissue. Blinded to the MRE results, neurosurgeons made a qualitative assessment of tumor stiffness at the time of resection. The ability of MRE to predict the surgical assessment of stiffness was tested using a Spearman rank correlation.One case was excluded due to a small tumor size. In the remaining 12 cases, both tumor stiffness alone (p = 0.023) and the ratio of tumor stiffness to surrounding brain tissue stiffness (p = 0.0032) significantly correlated with the surgeons' qualitative assessment of tumor stiffness. Results of the MRE examination provided a stronger correlation with the surgical assessment of stiffness compared with traditional T1- and T2-weighted imaging (p = 0.089), particularly when considering meningiomas of intermediate stiffness.In this cohort, preoperative MRE predicted tumor consistency at the time of surgery. Tumor stiffness as measured using MRE outperformed conventional MRI because tumor appearance on T1- and T2-weighted images could only accurately predict the softest and hardest meningiomas.
Non-invasive characterization of intracranial tumors by magnetic resonance elastography
[J].
Higher-resolution magnetic resonance elastography in meningiomas to determine intratumoral consistency
[J].
DOI:10.1227/NEU.0000000000000892
PMID:26197204
[本文引用: 2]

Magnetic resonance elastography (MRE) analyzes shear wave movement through tissue to determine stiffness. In a prior study, measurements with first-generation brain MRE techniques correlated with intraoperative observations of overall meningioma stiffness.To evaluate the diagnostic accuracy of a higher-resolution MRE technique to preoperatively detect intratumoral variations compared with surgeon assessment.Fifteen meningiomas in 14 patients underwent MRE. Tumors with regions of distinctly different stiffness were considered heterogeneous. Intratumoral portions were considered hard if there was a significant area ≥6 kPa. A 5-point scale graded intraoperative consistency. A durometer semiquantitatively measured surgical specimen hardness. Statistics included χ, sensitivity, specificity, positive and negative predicative values, and Spearman rank correlation coefficient.For MRE and surgery, 9 (60%) and 7 (47%) tumors were homogeneous, 6 (40%) and 8 (53%) tumors were heterogeneous, 6 (40%) and 10 (67%) tumors had hard portions, and 14 (93%) and 12 (80%) tumors had soft portions, respectively. MRE sensitivity, specificity, and positive and negative predictive values were as follows: for heterogeneity, 75%, 100%, 100%, and 87%; for hardness, 60%, 100%, 100%, and 56%; and for softness, 100%, 33%, 86%, and 100%. Overall, 10 tumors (67%) matched well with MRE and intraoperative consistency and correlated between intraoperative observations (P =.02) and durometer readings (P =.03). Tumor size ≤3.5 cm or vascular tumors were more likely to be inconsistent (P <.05).MRE was excellent at ruling in heterogeneity with hard portions but less effective in ruling out heterogeneity and hard portions, particularly in tumors more vascular or <3.5 cm. MRE is the first technology capable of prospectively evaluating intratumoral stiffness and, with further refinement, will likely prove useful in preoperative planning.
Magnetic resonance elastography detects tumoral consistency in pituitary macroadenomas
[J].
DOI:10.1007/s11102-016-0706-5
PMID:26782836
[本文引用: 2]

Most pituitary macroadenomas (PMA) are soft and suckable allowing transsphenoidal resection. A small percentage of PMA are firm, which significantly alters the time, technical difficulty, and effectiveness of transsphenoidal surgery. No current imaging technology can reliably assess PMA viscoelastic consistency in preparation for surgery. Magnetic resonance elastography (MRE) is an MRI-based technique that measures the propagation of mechanically induced shear waves through tissue to calculate stiffness. We prospectively evaluated MRE in 10 patients undergoing transsphenoidal resection of PMA to determine feasibility and potential usefulness.10 patients with PMA > 2.0 cm in maximum diameter were prospectively imaged with MRE prior to transsphenoidal surgery. Mean patient age was 59.5 ± 16.2 (22-78) years. Five were female and five male. MRE was performed with a modified single-shot spin-echo echo-planar-imaging pulse sequence on a 3T MRI. MRE values were independently calculated. The surgeon, blinded to the MRE results, graded tumor consistency at surgery as soft, intermediate, or firm. Chi-squared test compared surgical grading and MRE stiffness values.MRE was accomplished in all patients with excellent resolution. By surgical categorization, six tumors were soft and four intermediate. The mean MRE value for soft tumors was 1.38 ± 0.36 (1.08-1.87) kPa, while for intermediate tumors it was 1.94 ± 0.26 (1.72-2.32) kPa (p = 0.020).Determination of PMA stiffness is feasible with MRE. There was a statistically significant difference in MRE values between soft and intermediate PMAs. Further study in a larger series is ongoing to determine whether MRE will prove useful in preoperative planning for PMA.
MR elastography analysis of glioma stiffness and IDH1-mutation status
[J].
Predicting pituitary adenoma consistency with preoperative magnetic resonance elastography
[J].
Shear stiffness of 4 common intracranial tumors measured using MR elastography: comparison with intraoperative consistency grading
[J].
DOI:10.3174/ajnr.A4832
PMID:27339950
[本文引用: 2]

The stiffness of intracranial tumors affects the outcome of tumor removal. We evaluated the stiffness of 4 common intracranial tumors by using MR elastography and tested whether MR elastography had the potential to discriminate firm tumors preoperatively.Thirty-four patients with meningiomas, pituitary adenomas, vestibular schwannomas, and gliomas scheduled for resection were recruited for MR elastography. On the elastogram, the mean and the maximum shear stiffnesses were measured by placing an ROI on the tumor. Blinded to the MR elastography findings, surgeons conducted qualitative intraoperative assessment of tumor consistency by using a 5-point scale. Histopathologic diagnosis was confirmed by using the resected specimens. The mean and maximum shear stiffnesses were compared with histopathologic subtypes, and the intraoperative tumor consistency was graded by the surgeons.The mean and maximum shear stiffnesses were the following: 1.9 ± 0.8 kPa and 3.4 ± 1.5 kPa for meningiomas, 1.2 ± 0.3 kPa and 1.8 ± 0.5 kPa for pituitary adenomas, 2.0 ± 0.4 kPa and 2.7 ± 0.8 kPa for vestibular schwannomas, and 1.5 ± 0.2 kPa and 2.7 ± 0.8 kPa for gliomas. The mean and maximum shear stiffnesses for meningiomas were higher than those of pituitary adenomas (<.05). The mean and maximum shear stiffnesses were significantly correlated with the surgeon's qualitative assessment of tumor consistency (<.05). The maximum shear stiffness for 5 firm tumors was higher than that of nonfirm tumors (<.05).MR elastography could evaluate intracranial tumors on the basis of their physical property of shear stiffness. MR elastography may be useful in discriminating firm tumors preoperatively.© 2016 by American Journal of Neuroradiology.
Relationship between shear stiffness measured by MR elastography and perfusion metrics measured by perfusion CT of meningiomas
[J].
DOI:10.3174/ajnr.A7117
PMID:33985944
[本文引用: 2]

When managing meningiomas, intraoperative tumor consistency and histologic subtype are indispensable factors influencing operative strategy. The purposes of this study were the following: 1) to investigate the correlation between stiffness assessed with MR elastography and perfusion metrics from perfusion CT, 2) to evaluate whether MR elastography and perfusion CT could predict intraoperative tumor consistency, and 3) to explore the predictive value of stiffness and perfusion metrics in distinguishing among histologic subtypes of meningioma.Mean tumor stiffness and relative perfusion metrics (blood flow, blood volume, and MTT) were calculated (relative to normal brain tissue) for 14 patients with meningiomas who underwent MR elastography and perfusion CT before surgery (cohort 1). Intraoperative tumor consistency was graded by a neurosurgeon in 18 patients (cohort 2, comprising the 14 patients from cohort 1 plus 4 additional patients). The correlation between tumor stiffness and perfusion metrics was evaluated in cohort 1, as was the ability of perfusion metrics to predict intraoperative tumor consistency and discriminate histologic subtypes. Cohort 2 was analyzed for the ability of stiffness to determine intraoperative tumor consistency and histologic subtypes.The relative MTT was inversely correlated with stiffness ( =.006). Tumor stiffness was positively correlated with intraoperative tumor consistency ( =.01), while perfusion metrics were not. Relative MTT significantly discriminated transitional meningioma from meningothelial meningioma ( =.04), while stiffness did not significantly differentiate any histologic subtypes.In meningioma, tumor stiffness may be useful to predict intraoperative tumor consistency, while relative MTT may potentially correlate with tumor stiffness and differentiate transitional meningioma from meningothelial meningioma.© 2021 by American Journal of Neuroradiology.
Use of magnetic resonance elastography to gauge meningioma intratumoral consistency and histotype
[J].
High-resolution mechanical imaging of glioblastoma by multifrequency magnetic resonance elastography
[J].
High resolution imaging of viscoelastic properties of intracranial tumours by multi-frequency magnetic resonance elastography
[J].
Whole tissue and single cell mechanics are correlated in human brain tumors
[J].
DOI:10.1039/d1sm01291f
PMID:34787626
[本文引用: 2]

Biomechanical changes are critical for cancer progression. However, the relationship between the rheology of single cells measured ex-vivo and the living tumor is not yet understood. Here, we combined single-cell rheology of cells isolated from primary tumors with bulk tumor rheology in patients with brain tumors. Eight brain tumors (3 glioblastoma, 3 meningioma, 1 astrocytoma, 1 metastasis) were investigated by magnetic resonance elastography (MRE), and after surgery by the optical stretcher (OS). MRE was performed in a 3-Tesla clinical MRI scanner and magnitude modulus ||, loss angle, storage modulus ', and loss modulus '' were derived. OS experiments measured cellular creep deformation in response to laser-induced step stresses. We used a Kelvin-Voigt model to deduce two parameters related to cellular stiffness () and cellular viscosity () from OS measurements in a time regimen that overlaps with that of MRE. We found that single-cell was correlated with || ( = 0.962, < 0.001) and ( = 0.883, = 0.004) but not of the bulk tissue. These results suggest that single-cell stiffness affects tissue viscosity in brain tumors. The observation that viscosity parameters of individual cells and bulk tissue were not correlated suggests that collective mechanical interactions ( emergent effects or cellular unjamming) of many cancer cells, which depend on cellular stiffness, influence the mechanical dissipation behavior of the bulk tissue. Our results are important to understand the emergent rheology of active multiscale compound materials such as brain tumors and its role in disease progression.
Decreased tissue stiffness in glioblastoma by MR elastography is associated with increased cerebral blood flow
[J].
The impact of aging and gender on brain viscoelasticity
[J].
DOI:10.1016/j.neuroimage.2009.02.040
PMID:19281851
[本文引用: 1]

Viscoelasticity is a sensitive measure of the microstructural constitution of soft biological tissue and is increasingly used as a diagnostic marker, e.g. in staging liver fibrosis or characterizing breast tumors. In this study, multifrequency magnetic resonance elastography was used to investigate the in vivo viscoelasticity of healthy human brain in 55 volunteers (23 females) ranging in age from 18 to 88 years. The application of four vibration frequencies in an acoustic range from 25 to 62.5 Hz revealed for the first time how physiological aging changes the global viscosity and elasticity of the brain. Using the rheological springpot model, viscosity and elasticity are combined in a parameter mu that describes the solid-fluid behavior of the tissue and a parameter alpha related to the tissue's microstructure. It is shown that the healthy adult brain undergoes steady parenchymal 'liquefaction' characterized by a continuous decline in mu of 0.8% per year (P<0.001), whereas alpha remains unchanged. Furthermore, significant sex differences were found with female brains being on average 9% more solid-like than their male counterparts rendering women more than a decade 'younger' than men with respect to brain mechanics (P=0.016). These results set the background for using cerebral multifrequency elastography in diagnosing subtle neurodegenerative processes not detectable by other diagnostic methods.
Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults
[J].
DOI:10.1016/j.neuroimage.2015.02.016
PMID:25698157
[本文引用: 1]

Changes in tissue composition and cellular architecture have been associated with neurological disease, and these in turn can affect biomechanical properties. Natural biological factors such as aging and an individual's sex also affect underlying tissue biomechanics in different brain regions. Understanding the normal changes is necessary before determining the efficacy of stiffness imaging for neurological disease diagnosis and therapy monitoring. The objective of this study was to evaluate global and regional changes in brain stiffness as a function of age and sex, using improved MRE acquisition and processing that have been shown to provide median stiffness values that are typically reproducible to within 1% in global measurements and within 2% for regional measurements. Furthermore, this is the first study to report the effects of age and sex over the entire cerebrum volume and over the full frontal, occipital, parietal, temporal, deep gray matter/white matter (insula, deep gray nuclei and white matter tracts), and cerebellum volumes. In 45 volunteers, we observed a significant linear correlation between age and brain stiffness in the cerebrum (P<.0001), frontal lobes (P<.0001), occipital lobes (P=.0005), parietal lobes (P=.0002), and the temporal lobes (P<.0001) of the brain. No significant linear correlation between brain stiffness and age was observed in the cerebellum (P=.74), and the sensory-motor regions (P=.32) of the brain, and a weak linear trend was observed in the deep gray matter/white matter (P=.075). A multiple linear regression model predicted an annual decline of 0.011 ± 0.002 kPa in cerebrum stiffness with a theoretical median age value (76 years old) of 2.56 ± 0.08 kPa. Sexual dimorphism was observed in the temporal (P=.03) and occipital (P=.001) lobes of the brain, but no significant difference was observed in any of the other brain regions (P>.20 for all other regions). The model predicted female occipital and temporal lobes to be 0.23 kPa and 0.09 kPa stiffer than males of the same age, respectively. This study confirms that as the brain ages, there is softening; however, the changes are dependent on region. In addition, stiffness effects due to sex exist in the occipital and temporal lobes.Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Effect of aging on the viscoelastic properties of hippocampal subfields assessed with high-resolution MR elastography
[J].
Hippocampal subfield viscoelasticity in amnestic mild cognitive impairment evaluated with MR elastography
[J].
Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography
[J].
DOI:10.1002/jmri.22707
PMID:21751286
[本文引用: 2]

To test patient acceptance and reproducibility of the 3D magnetic resonance elastography (MRE) brain exam using a soft vibration source, and to determine if MRE could noninvasively measure a change in the elastic properties of the brain parenchyma due to Alzheimer's disease (AD).MRE exams were performed using an accelerated spin-echo echo planar imaging (EPI) pulse sequence and stiffness was calculated with a 3D direct inversion algorithm. Reproducibility of the technique was assessed in 10 male volunteers, who each underwent four MRE exams separated into two imaging sessions. The effect of AD on brain stiffness was assessed in 28 volunteers, 7 with probable AD, 14 age- and gender-matched PIB-negative (Pittsburgh Compound B, a PET amyloid imaging ligand) cognitively normal controls (CN-), and 7 age- and gender-matched PIB-positive cognitively normal controls (CN+).The median stiffness of the 10 volunteers was 3.07 kPa with a range of 0.40 kPa. The median and maximum coefficients of variation for these volunteers were 1.71% and 3.07%. The median stiffness of the 14 CN- subjects was 2.37 kPa (0.44 kPa range) compared to 2.32 kPa (0.49 kPa range) within the CN+ group and 2.20 kPa (0.33 kPa range) within the AD group. A significant difference was found between the three groups (P = 0.0055, Kruskal-Wallis one-way analysis of variance). Both the CN+ and CN- groups were significantly different from the AD group.3D MRE of the brain can be performed reproducibly and demonstrates significantly reduced brain tissue stiffness in patients with AD.Copyright © 2011 Wiley-Liss, Inc.
Regional brain stiffness changes across the Alzheimer's disease spectrum
[J].
Differential effect of dementia etiology on cortical stiffness as assessed by MR elastography
[J].
Combining viscoelasticity, diffusivity and volume of the hippocampus for the diagnosis of Alzheimer’s disease based on magnetic resonance imaging
[J].
Mechanical property alterations across the cerebral cortex due to Alzheimer’s disease
[J].
Progressive supranuclear palsy and idiopathic Parkinson’s disease are associated with local reduction of in vivo brain viscoelasticity
[J].
In vivo waveguide elastography: Effects of neurodegeneration in patients with amyotrophic lateral sclerosis
[J].
DOI:10.1002/mrm.25067
PMID:24347290
[本文引用: 2]

Waveguide elastography (WGE) combines magnetic resonance elastography (MRE), diffusion tensor imaging (DTI), and anisotropic inversions for a determination of the elastic properties of white matter. Previously, the method evaluated the anisotropic elastic properties of the corticospinal tracts (CSTs) of healthy volunteers. Here, the sensitivity of WGE is tested for the detection of pathologic changes in a cohort of patients with Amyotrophic Lateral Sclerosis (ALS).MRE and DTI were performed in 14 patients with ALS and 14 healthy, age-matched controls. A comparison was made between three components from WGE and the DTI metrics FA, MD, PD, and RD, for the detection of differences between patients and controls. It was hypothesized that the stiffness values in the CSTs of the patients would be significantly lower due to the known neurodegeneration associated with ALS.Two anisotropic shear moduli polarized parallel and perpendicular to the CSTs were significantly reduced in ALS patients (P < 0.0001), whereas the anisotropic longitudinal modulus polarized parallel to the CSTs showed no significant differences.The results of this study suggest a relatively high sensitivity of two anisotropic shear moduli as noninvasive metrics for the assessment of neuronal degeneration within the CSTs.© 2013 Wiley Periodicals, Inc.
Magnetic resonance elastography of frontotemporal dementia
[J].
DOI:10.1002/jmri.24977
PMID:26130216
[本文引用: 2]

To investigate the feasibility of utilizing brain stiffness as a potential biomarker for behavioral variant frontotemporal dementia (bvFTD) patients. Magnetic resonance elastography (MRE) is a noninvasive technique for evaluating the mechanical properties of brain tissue in vivo. MRE has demonstrated decreased brain stiffness in patients with Alzheimer's disease.We examined five male subjects with bvFTD and nine cognitively normal age-matched male controls (NC) with brain 3T MRE. Stiffness was calculated in nine regions of interest (ROIs): whole brain (entire cerebrum excluding cerebellum), frontal lobes, occipital lobes, parietal lobes, temporal lobes, deep gray matter / white matter (GM/WM; insula, deep gray nuclei and white matter tracts), cerebellum, sensorimotor cortex (pre- and postcentral gyri), and a composite region labeled FT (frontal and temporal lobes excluding the pre- and postcentral gyri).Significantly lower stiffness values were observed in the whole brain (P = 0.007), frontal lobe (P = 0.001), and temporal lobes (P = 0.005) of bvFTD patients compared to NC. No significant stiffness differences were observed in any other ROIs of bvFTD patients compared to NC (P > 0.05). These results demonstrate that statistically significant brain softening occurs in the frontal and temporal lobes of bvFTD patients, which corresponds to the expected pathophysiology of bvFTD.Future studies evaluating the feasibility of brain MRE for early disease detection and monitoring disease progression could shed new insights into understanding the mechanisms involved in bvFTD.© 2015 The Authors Journal of Magnetic Resonance Imaging published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
MR elastography demonstrates unique regional brain stiffness patterns in dementias
[J].
DOI:10.2214/AJR.16.17455
PMID:28570101
[本文引用: 2]

The purpose of this study was to investigate age-corrected brain MR elastography (MRE) findings in four dementia cohorts (Alzheimer disease, dementia with Lewy bodies, frontotemporal dementia, and normal pressure hydrocephalus) and determine the potential use as a differentiating biomarker in dementia subtypes.Institutional review board approval and written informed consent were obtained to perform MRE on 84 subjects: 20 patients with normal pressure hydrocephalus, eight with Alzheimer disease, five with dementia with Lewy bodies, five with frontotemporal dementia, and 46 cognitively normal control subjects. Shear waves of 60-Hz vibration frequency were transmitted into the brain using a pillowlike passive driver, and brain stiffness was determined in eight different regions (cerebrum, frontal, occipital, parietal, temporal, deep gray matter-white matter, sensorimotor cortex, and cerebellum). All stiffness values were age-corrected and compared with control subjects. The Wilcoxon rank sum test and linear regression were used for statistical analysis.Regional stiffness patterns unique to each dementing disorder were observed. Patients with Alzheimer disease and frontotemporal dementia showed decreased cerebral stiffness (p = 0.001 and p = 0.002, respectively) with regional softening of the frontal and temporal lobes. Patients with Alzheimer disease additionally showed parietal lobe and sensorimotor region softening (p = 0.039 and p = 0.018, respectively). Patients with normal pressure hydrocephalus showed stiffening of the parietal, occipital, and sensorimotor regions (p = 0.007, p < 0.001, and p < 0.0001, respectively). Patients with dementia with Lewy bodies did not show significant stiffness changes in any of the regions.Quantitative MRE of changes in brain viscoelastic structure shows unique regional brain stiffness patterns between common dementia subtypes.
A generalized series approach to MR spectroscopic imaging
[J].
An efficient method for dynamic magnetic resonance imaging
[J].
Spatiotemporal Imaging with partially separable functions
[C]//
OSCILLATE: A low-rank approach for accelerated magnetic resonance elastography
[J].
DOI:10.1002/mrm.29308
PMID:35649188
[本文引用: 1]

MR elastography (MRE) is a technique to characterize brain mechanical properties in vivo. Due to the need to capture tissue deformation in multiple directions over time, MRE is an inherently long acquisition, which limits achievable resolution and use in challenging populations. The purpose of this work is to develop a method for accelerating MRE acquisition by using low-rank image reconstruction to exploit inherent spatiotemporal correlations in MRE data.The proposed MRE sampling and reconstruction method, OSCILLATE (Observing Spatiotemporal Correlations for Imaging with Low-rank Leveraged Acceleration in Turbo Elastography), involves alternating which k-space points are sampled between each repetition by a reduction factor, R Using a predetermined temporal basis from a low-resolution navigator in a joint low-rank image reconstruction, all images can be accurately reconstructed from a reduced amount of k-space data.Decomposition of MRE displacement data demonstrated that, on average, 96.1% of all energy from an MRE dataset is captured at rank L = 12 (reduced from a full rank of 24). Retrospectively undersampling data with R = 2 and reconstructing at low-rank (L = 12) yields highly accurate stiffness maps with voxel-wise error of 5.8% ± 0.7%. Prospectively undersampled data at R = 2 were successfully reconstructed without loss of material property map fidelity, with average global stiffness error of 1.0% ± 0.7% compared to fully sampled data.OSCILLATE produces whole-brain MRE data at 2 mm isotropic resolution in 1 min 48 s.© 2022 International Society for Magnetic Resonance in Medicine.
Fast 3D MR elastography of the whole brain using spiral staircase: Data acquisition, image reconstruction, and joint deblurring
[J].
DOI:10.1002/mrm.28855
PMID:34096097
[本文引用: 1]

To address the need for a method to acquire 3D data for MR elastography (MRE) of the whole brain with substantially improved spatial resolution, high SNR, and reduced acquisition time compared with conventional methods.We combined a novel 3D spiral staircase data-acquisition method with a spoiled gradient-echo pulse sequence and MRE motion-encoding gradients (MEGs). The spiral-out acquisition permitted use of longer-duration motion-encoding gradients (ie, over two full oscillatory cycles) to enhance displacement SNR, while still maintaining a reasonably short TE for good phase-SNR. Through-plane parallel imaging with low noise penalties was implemented to accelerate acquisition along the slice direction. Shared anatomical information was exploited in the deblurring procedure to further boost SNR for stiffness inversion.In vivo and phantom experiments demonstrated the feasibility of the proposed method in producing brain MRE results comparable to the spin-echo-based approaches, both qualitatively and quantitatively. High-resolution (2-mm isotropic) brain MRE data were acquired in 5 minutes using our method with good SNR. Joint deblurring with shared anatomical information produced SNR-enhanced images, leading to upward stiffness estimation.A novel 3D gradient-echo-based approach has been designed and implemented, and shown to have promising potential for fast and high-resolution in vivo MRE of the whole brain.© 2021 International Society for Magnetic Resonance in Medicine.
TURBINE-MRE: A 3D hybrid radial-Cartesian EPI acquisition for MR elastography
[J].
Imaging brain function with simultaneous BOLD and viscoelasticity contrast: fMRI/fMRE
[J].
Concurrent 3D acquisition of diffusion tensor imaging and magnetic resonance elastography displacement data (DTI-MRE): Theory and in vivo application
[J].
DOI:10.1002/mrm.26121
PMID:26787007
[本文引用: 1]

To introduce a newly developed technique (DTI-MRE) for the simultaneous acquisition of diffusion tensor imaging (DTI) and 3D-vector field magnetic resonance elastography (MRE) data, and to demonstrate its feasibility when applied in vivo to the mouse brain.In DTI-MRE, simultaneous encoding is achieved by using a series of diffusion/motion-sensitizing gradients (dMSGs) with specific timing and directions. By adjusting the duration of the dMSGs with the diffusion time and with the mechanical vibration frequency, the shear wave motion and diffusion are encoded into the MR phase and MR magnitude signals, respectively. The dMSGs are applied in a noncollinear and noncoplanar manner that optimizes the capture of both the DTI signal attenuation and the three-dimensional MRE displacements. In this work, the feasibility of the DTI-MRE technique was demonstrated on in vivo mouse brains (n=3) using a 9.4T animal MRI scanner. The DTI-MRE derived parameters (MD, mean diffusivity; FA, fractional anisotropy; MRE displacement fields; and shear modulus |G|) were compared with those acquired using conventional, separate MRE and diffusion methods.The averaged (MD, FA, and |G|) values for three mice are (0.580 ± 0.050 µm /ms, 0.43 ± 0.02, and 4.80 ± 0.06 kPa) and (0.583 ± 0.035 µm /ms, 0.46 ± 0.02, and 4.91 ± 0.19 kPa) for DTI-MRE, and conventional DTI and 3D-vector field MRE measurements, respectively. All derived parameters (MD, FA, |G|, and displacement) obtained using the combined DTI-MRE method and conventional methods were significantly correlated with P < 0.05.Simultaneous acquisition of DTI and 3D-vector field MRE is feasible in vivo and reduces the scan time by up to 50% compared with conventional, separate acquisitions, while providing an immediate co-registration of maps of diffusion properties and stiffness. Magn Reson Med 77:273-284, 2017. © 2016 Wiley Periodicals, Inc.© 2016 Wiley Periodicals, Inc.
Asynchronous magnetic resonance elastography: Shear wave speed reconstruction using noise correlation of incoherent waves
[J].
Brain palpation from physiological vibrations using MRI
[J].
Standard-space atlas of the viscoelastic properties of the human brain
[J].
Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter
[J].
DOI:10.1016/j.jmbbm.2013.04.007
PMID:23680651
[本文引用: 2]

White matter in the brain is structurally anisotropic, consisting largely of bundles of aligned, myelin-sheathed axonal fibers. White matter is believed to be mechanically anisotropic as well. Specifically, transverse isotropy is expected locally, with the plane of isotropy normal to the local mean fiber direction. Suitable material models involve strain energy density functions that depend on the I4 and I5 pseudo-invariants of the Cauchy-Green strain tensor to account for the effects of relatively stiff fibers. The pseudo-invariant I4 is the square of the stretch ratio in the fiber direction; I5 contains contributions of shear strain in planes parallel to the fiber axis. Most, if not all, published models of white matter depend on I4 but not on I5. Here, we explore the small strain limits of these models in the context of experimental measurements that probe these dependencies. Models in which strain energy depends on I4 but not I5 can capture differences in Young's (tensile) moduli, but will not exhibit differences in shear moduli for loading parallel and normal to the mean direction of axons. We show experimentally, using a combination of shear and asymmetric indentation tests, that white matter does exhibit such differences in both tensile and shear moduli. Indentation tests were interpreted through inverse fitting of finite element models in the limit of small strains. Results highlight that: (1) hyperelastic models of transversely isotropic tissues such as white matter should include contributions of both the I4 and I5 strain pseudo-invariants; and (2) behavior in the small strain regime can usefully guide the choice and initial parameterization of more general material models of white matter.Copyright © 2013 Elsevier Ltd. All rights reserved.
Characterizing white matter tissue in large strain via asymmetric indentation and inverse finite element modeling
[J].
DOI:S1751-6161(16)30326-5
PMID:27665084
[本文引用: 1]

Characterizing the mechanical properties of white matter is important to understand and model brain development and injury. With embedded aligned axonal fibers, white matter is typically modeled as a transversely isotropic material. However, most studies characterize the white matter tissue using models with a single anisotropic invariant or in a small-strain regime. In this study, we combined a single experimental procedure - asymmetric indentation - with inverse finite element (FE) modeling to estimate the nearly incompressible transversely isotropic material parameters of white matter. A minimal form comprising three parameters was employed to simulate indentation responses in the large-strain regime. The parameters were estimated using a global optimization procedure based on a genetic algorithm (GA). Experimental data from two indentation configurations of porcine white matter, parallel and perpendicular to the axonal fiber direction, were utilized to estimate model parameters. Results in this study confirmed a strong mechanical anisotropy of white matter in large strain. Further, our results suggested that both indentation configurations are needed to estimate the parameters with sufficient accuracy, and that the indenter-sample friction is important. Finally, we also showed that the estimated parameters were consistent with those previously obtained via a trial-and-error forward FE method in the small-strain regime. These findings are useful in modeling and parameterization of white matter, especially under large deformation, and demonstrate the potential of the proposed asymmetric indentation technique to characterize other soft biological tissues with transversely isotropic properties.Copyright © 2016 Elsevier Ltd. All rights reserved.
Microscale characterisation of the time-dependent mechanical behaviour of brain white matter
[J].
Identification of normal pressure hydrocephalus by disease-specific patterns of brain stiffness and damping ratio
[J].
DOI:10.1097/RLI.0000000000000630
PMID:32058331
[本文引用: 1]

The aim of this study was to perform a whole-brain analysis of alterations in brain mechanical properties due to normal pressure hydrocephalus (NPH).Magnetic resonance elastography (MRE) examinations were performed on 85 participants, including 44 cognitively unimpaired controls, 33 with NPH, and 8 who were amyloid-positive with Alzheimer clinical syndrome. A custom neural network inversion was used to estimate stiffness and damping ratio from patches of displacement data, accounting for edges by training the network to estimate the mechanical properties in the presence of missing data. This learned inversion was first compared with a standard analytical approach in simulation experiments and then applied to the in vivo MRE measurements. The effect of NPH on the mechanical properties was then assessed by voxel-wise modeling of the stiffness and damping ratio maps. Finally, a pattern analysis was performed on each individual's mechanical property maps by computing the correlation between each person's maps with the expected NPH effect. These features were used to fit a classifier and assess diagnostic accuracy.The voxel-wise analysis of the in vivo mechanical property maps revealed a unique pattern in participants with NPH, including a concentric pattern of stiffening near the dural surface and softening near the ventricles, as well as decreased damping ratio predominantly in superior regions of the white matter (family-wise error corrected P < 0.05 at cluster level). The pattern of viscoelastic changes in each participant predicted NPH status in this cohort, separating participants with NPH from the control and the amyloid-positive with Alzheimer clinical syndrome groups, with areas under the receiver operating characteristic curve of 0.999 and 1, respectively.This study provides motivation for further development of the neural network inversion framework and demonstrates the potential of MRE as a novel tool to diagnose NPH and provide a window into its pathogenesis.
Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography
[J].
DOI:10.1002/mrm.20355
PMID:15678538
[本文引用: 1]

MR-elastography is a new technique for assessing the viscoelastic properties of tissue. One current focus of elastography is the provision of new physical parameters for improving the specificity in breast cancer diagnosis. This analysis describes a technique to extend the reconstruction to anisotropic elastic properties in terms of a so-called transversely isotropic model. Viscosity is treated as being isotropic. The particular model chosen for the anisotropy is appealing because it is capable of describing elastic shear anisotropy of parallel fibers. The dependence of the reconstruction on the particular choice of Poisson's ratio is eliminated by extracting the compressional displacement contribution using the Helmholtz-Hodge decomposition. Results are presented for simulations, a polyvinyl alcohol breast phantom, excised beef muscle, and measurements in two patients with breast lesions (invasive ductal carcinoma and fibroadenoma). The results show enhanced anisotropic and viscous properties inside the lesions and an indication for preferred fiber orientation.
In vivo waveguide elastography of white matter tracts in the human brain
[J].
DOI:10.1002/mrm.24141
PMID:22252792
[本文引用: 1]

White matter is composed primarily of myelinated axons which form fibrous, organized structures and can act as waveguides for the anisotropic propagation of sound. The evaluation of their elastic properties requires both knowledge of the orientation of these waveguides in space, as well as knowledge of the waves propagating along and through them. Here, we present waveguide elastography for the evaluation of the elastic properties of white matter tracts in the human brain, in vivo, using a fusion of diffusion tensor imaging, magnetic resonance elastography, spatial-spectral filtering, a Helmholtz decomposition, and anisotropic inversions, and apply this method to evaluate the material parameters of the corticospinal tracts of five healthy human volunteers. We begin with an Orthotropic inversion model and demonstrate that redundancies in the solution for the nine elastic coefficients indicate that the corticospinal tracts can be approximated by a Hexagonal model (transverse isotropy) comprised of five elastic coefficients representative of a medium with fibers aligned parallel to a central axis, and provides longitudinal and transverse wave velocities on the order of 5.7 m/s and 2.1 m/s, respectively. This method is intended as a new modality to assess white matter structure and health by means of the evaluation of the anisotropic elasticity tensor of nerve fibers.Copyright © 2012 Wiley Periodicals, Inc.
Mapping heterogenous anisotropic tissue mechanical properties with transverse isotropic nonlinear inversion MR elastography
[J].
Transversely-isotropic brain in vivo MR elastography with anisotropic damping
[J].
Magnetic resonance elastography reconstruction for anisotropic tissues
[J].
Magnetic resonance elastography of brain: Comparison between anisotropic and isotropic stiffness and its correlation to age
[J].
DOI:10.1002/mrm.27757
PMID:30957304
[本文引用: 1]

Noninvasive measurement of mechanical properties of brain tissue using magnetic resonance elastography (MRE) has been a promising method for investigating neurologic disorders such as multiple sclerosis, hydrocephalus, and Alzheimer's. However, because of the regional and directional dependency of brain stiffness, estimating anisotropic stiffness is important. This study investigates isotropic and anisotropic stiffness as a function of age as well as the correlation between isotropic and anisotropic stiffness.MRE and diffusion tensor imaging (DTI) were performed on 28 healthy subjects with age ranges between 18-62 y. Isotropic and anisotropic stiffness was measured and compared with age for different regions of interest such as the thalamus, corpus callosum, gray matter, white matter, and whole brain.Isotropic stiffness in gray matter (r = -0.57; P = 0.001) showed a significant decrease with age. Anisotropic stiffness in gray matter showed a significant decrease with age in C through C and in the thalamus, only in C. Between anisotropic and isotropic stiffness, gray matter showed a significant positive correlation in C through C, C and C showed a significant negative correlation in the thalamus and whole brain, and C showed a negative correlation in the corpus callosum. No significant difference between genders was observed in any measurements.This study demonstrated a change in isotropic and anisotropic stiffness with age in different regions of the brain along with a correlation of anisotropic stiffness to isotropic stiffness.© 2019 International Society for Magnetic Resonance in Medicine.
Estimation of the mechanical properties of a transversely isotropic material from shear wave fields via artificial neural networks
[J].
Diffusion and intravoxel incoherent motion MR imaging-based virtual elastography: A hypothesis-generating study in the liver
[J].
DOI:10.1148/radiol.2017170025
PMID:28604279
[本文引用: 1]

Purpose To investigate the potential of diffusion magnetic resonance (MR) imaging to provide quantitative estimates of tissue stiffness without using mechanical vibrations in patients with chronic liver diseases and to generate a new elasticity-driven intravoxel incoherent motion (IVIM) contrast. Materials and Methods This retrospective study, conducted from January to April 2016, was approved by an institutional review board that waived the requirement for informed consent. Fifteen subjects were included (13 men and two women; mean age ± standard deviation, 73 years ± 8). MR elastography and diffusion MR imaging were performed at 3 T. A search for an empirical relationship between MR elastographic shear modulus, µ, and a shifted apparent diffusion coefficient (sADC) was performed. The sADC was then inverted to estimate patient liver shear modulus directly from diffusion MR imaging signals. Results A significant correlation (r = 0.90, P = 1 · 10) was observed between µ and sADC calculated by using diffusion MR imaging signals acquired with b values of 200 (S) and 1500 (S ) sec/mm (sACD). On the basis of the relationship between the µ and sADC, a diffusion-based shear modulus, µ, could be estimated with the following equation: µ = (-9.8 ± 0.8) ln(S/S) + (14.0 ± 0.9). IVIM virtual elastograms also could be generated to reveal new contrast features in lesions, depending on pseudovibration frequency and amplitude. Conclusion Diffusion MR imaging, through a calibration of sADC with standard MR elastography, can be converted quantitatively into shear modulus without using mechanical vibrations to provide information on the degree of liver fibrosis; these virtual elastograms require only two b values to be acquired and processed. Propagating shear wave can also be emulated, leading to a new elasticity-driven IVIM contrast with ranges of virtual vibration frequencies and amplitudes not limited by MR elastography or MR imaging hardware capacities. RSNA, 2017 Online supplemental material is available for this article.
Diffusion-weighted MRI-based virtual elastography for the assessment of liver fibrosis
[J].
Virtual magnetic resonance elastography has the feasibility to evaluate preoperative pituitary adenoma consistency
[J].
DOI:10.1007/s11102-021-01129-4
PMID:33555485
[本文引用: 1]

To evaluate the use of preoperative virtual Magnetic Resonance Elastography (vMRE) for patients undergoing transsphenoidal resection of pituitary adenomas (PA).Ten patients (60.2 ± 19.6 years; 8 males) were prospectively examined with the vMRE-method prior to transsphenoidal surgery. vMRE-images, reflecting tissue stiffness were reconstructed. From these images, histograms as well as the mean stiffness values over the tumor body were extracted. Finally, vMRE-data was compared with the PA consistency at surgery blinded to vMRE.In all patients, successful vMRE-examination was performed enabling evaluation of even small PAs. For tumors with homogenous tissue, the mean stiffness value increased with surgical consistency grading. For heterogenous tumors, however, the mean stiffness value did not consistently reflect the grading at surgery. On the other hand, the vMRE-images and histograms were found to be able to characterize the tumor heterogeneity and display focal regions of high stiffness that were found to affect the surgery outcome in these PAs. The vMRE-images and histograms showed great promise in characterizing the consistency at surgery for these PAs.Evaluation of PA consistency in preparation for surgery seems to be feasible using the vMRE-method. Our findings also address the need for high resolution diagnostic methods that can non-invasively display focal regions of increased stiffness, as such regions may increase the difficulty of transsphenoidal PA-resection.© 2021. The Author(s).
Global mapping of live cell mechanical features using PeakForce QNM AFM
[J].
A study of diagnostic performance of MR elastography in liver fibrosis with chronic hepatitis B
[J].
MR弹性成像对慢性乙型肝炎肝纤维化的诊断价值
[J].
Application and research progress of magnetic resonance elastography in cancer
[J].
磁共振弹性成像技术在肿瘤中的应用及研究进展
[J].
Brain structure and structural basis of neurodegenerative diseases
[J].
Cellular and animal models to investigate pathogenesis of amyloid aggregation in neurodegenerative diseases
[J].