蛋白质二硫键异构酶与α-突触核蛋白的相互作用及对其聚集的影响
Inhibition of α-Synuclein Aggregation by the Interaction Between Protein Disulfide Isomerase and α-Synuclein
蛋白质二硫键异构酶与α-突触核蛋白的相互作用及对其聚集的影响 |
| 裴云山,张偲,刘晓黎,成凯,张则婷,李从刚 |
|
Inhibition of α-Synuclein Aggregation by the Interaction Between Protein Disulfide Isomerase and α-Synuclein |
| Yun-shan PEI,Cai ZHANG,Xiao-li LIU,Kai CHENG,Ze-ting ZHANG,Cong-gang LI |
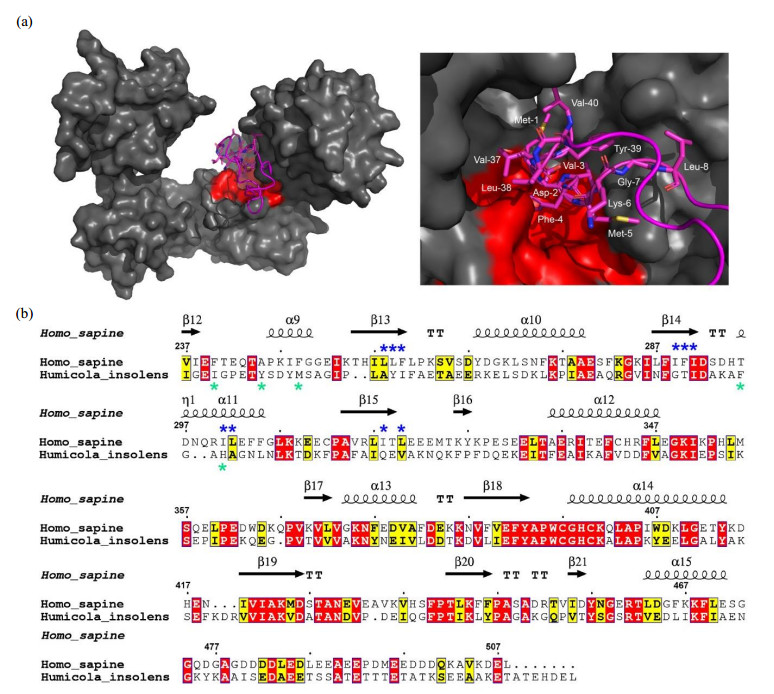
| 图4 PDI与αsyn N端的疏水结合模式. (a) PDI结合αsyn 1-40多肽的对接模型(左)及其结合区域的局部放大图(右),HDOCK对接实验在网站服务器(http://hdock.phys.hust.edu.cn/)上运行,上传人源PDI晶体结构,αsyn1-40序列及NMR滴定鉴定的结合位点,最佳对接结果使用PyMol软件作图,PDI表面结构呈灰色,疏水口袋以红色标出,αsyn1-40卡通结构呈品红色,右图中参与结合的αsyn残基以棒状模式显示并标出.(b)人源PDI b'xa'与HiPDI b'xa'的氨基酸序列比对.序列一致性和相似性由Clustalw[ |
| Fig.4 Hydrophobic binding mode of PDI with the N-terminal domain of αsyn. (a) Docking model of PDI binding αsyn1-40 (left panel) and a local zoom view of binding area (right panel). The relevant residues of αsyn1-40 involving in binding PDI were depicted as magenta sticks. HDOCK experiments were performed on the web server (http://hdock.phys.hust.edu.cn/) by uploading human PDI crystal structure, amino acid sequences of αsyn1-40 and their binding sites identified by NMR titration. Optimal docking results were plotted with PyMol software, PDI molecular surface was colored gray and its hydrophobic pocket was marked in red, the magenta cartoon represented αsyn1-40 structure. (b) Alignment of amino acid sequence of human PDI b'xa' from HiPDI b'xa'. Sequence identity and similarity were calculated by Clustalw[ |

|