蛋白质二硫键异构酶与α-突触核蛋白的相互作用及对其聚集的影响
Inhibition of α-Synuclein Aggregation by the Interaction Between Protein Disulfide Isomerase and α-Synuclein
蛋白质二硫键异构酶与α-突触核蛋白的相互作用及对其聚集的影响 |
| 裴云山,张偲,刘晓黎,成凯,张则婷,李从刚 |
|
Inhibition of α-Synuclein Aggregation by the Interaction Between Protein Disulfide Isomerase and α-Synuclein |
| Yun-shan PEI,Cai ZHANG,Xiao-li LIU,Kai CHENG,Ze-ting ZHANG,Cong-gang LI |
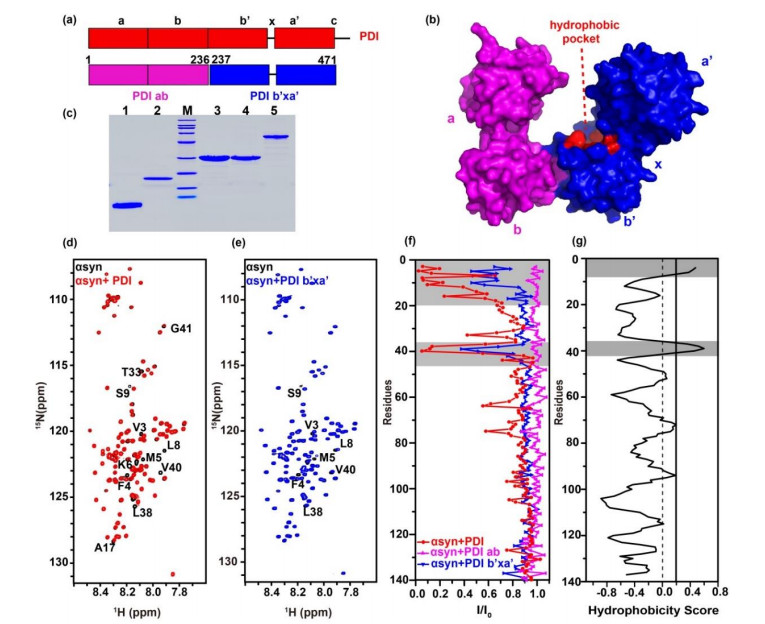
| 图1 PDI的b'xa'结构域与αsyn的N端区域相互作用. PDI(红色)的(a)一级序列模型及(b)晶体结构(PDB ID: 4EKZ)表面图,粉色和蓝色分别代表PDI ab与PDI b'xa'结构域;(c) SDS-PAGE分析各蛋白纯度,1~5泳道分别代表αsyn1-60、αsyn、PDI ab、PDI b'xa'、PDI,M代表Marker;(d)、(e) αsyn不加(黑色)与加入等量PDI(红色)或PDI b'xa'(蓝色)的谱图叠加,图中标注了明显发生信号减弱或移动的残基;(f)加入等量PDI(红色)、PDI ab(粉色)、PDI b'xa'(蓝色)后,αsyn谱峰强度变化(I/I0);(g)基于OMH法预测αsyn序列疏水性分析图,互作区域或疏水性较强的区域用灰色标出 |
| Fig.1 The b'xa' domain of PDI interacts with N-terminal domain of αsyn. (a) Model for PDI (red) primary sequence, PDI ab domain (pink) and PDI b'xa' domain (blue) are shown underneath. (b) Surface crystal structure of human PDI (PDB ID: 4EKZ). (c) Protein purities analyzed by SDS-PAGE. M, Marker; lane 1, αsyn1-60; lane 2, αsyn; lane 3, PDI ab; lane 4, PDI b'xa'; lane 5, PDI. (d) and (e) Spectral overlay of αsyn in the absence (black) and presence of equivalent amount of PDI (red) and PDI b'xa' (blue). Residues showing significant signal attenuation or shifts were marked. (f) Residue-resolved attenuation (I/I0) of αsyn in the presence of PDI (red), PDI ab (pink) and PDI b'xa' (blue). (g) Hydrophobic residues of αsyn predicted by OMH method, the binding and hydrophobic areas are colored gray |

|