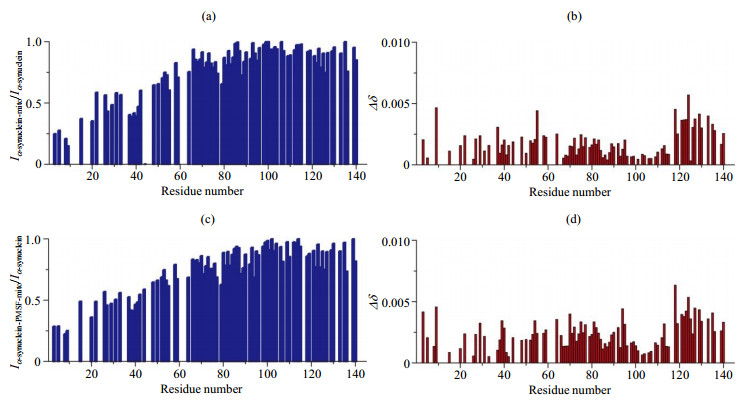
图3. α-synuclein线粒体样品氨基酸残基信号强度与化学位移变化结果. 相比于稀溶液,α-synuclein线粒体样品氨基酸残基的(a)强度变化和(b)化学位移变化;相比于稀溶液,α-synuclein-PMSF线粒体样品氨基酸残基的(c)强度变化和(d)化学位移变化(注:图中没有数值的空白部分主要为因谱峰重叠严重导致难以获取准确信号强度的氨基酸残基)
Fig.3. The analysis of residue resolved NMR signal intensity and chemical shift change of α-synuclein in the presence of mitochondria (the values of NMR signal intensity ratio and chemical shift changes are shown as blank for the residues whose signal intensity can not be accurately obtained due to the overlap of spectral peaks). (a) NMR signal intensity ratio (Iα-synuclein-mito/Iα-synuclein) of α-synuclein in the presence and absence of mitochondria; (b) Chemical shift changes of α-synuclein in presence of mitochondria compared to α-synuclein; (c) NMR signal intensity ratio (Iα-synuclein-PMSF-mito/Iα-synuclein) of α-synuclein with PMSF in the presence of mitochondria and α-synuclein; (d) Chemical shift changes of α-synuclein with PMSF in the presence of mitochondria compared to α-synuclein